The COVID-19 pandemic is in its 21st month. Vaccines have been developed, several strains have emerged, and millions of people have lost their lives due to the disease. However, a standard therapeutic regimen to counter the SARS-CoV-2 virus and manage viral loads in the infected remains elusive. Offering some hope, a new study has found that a drug used to treat abnormal levels of fatty substances (cholesterol and lipids) in the blood could reduce infection by up to 70 percent.
An international study has shown that fenofibrate—an approved and affordable drug—and fenofibric acid (its active form) can considerably reduce the novel coronavirus infection in human cells. Particularly, the decrease in the level of infection was achieved at concentrations that were safe and attainable with a standard clinical dose of the drug. The research was published in the journal Frontiers in Pharmacology.
"Given that fenofibrate is an oral drug which is very cheap and available worldwide, together with its extensive history of clinical use and its good safety profile, our data has global implications - especially in low-middle income countries and in those individuals for whom vaccines are not recommended or suitable such as children, those with hyper-immune disorders and those using immune-suppressants," said Dr. Elisa Vicenzi, co-author of the study, in a statement.
Helping Manage Cholesterol and Fight COVID-19?

Since the onset of the COVID-19 pandemic, existing compounds have been considered by scientists to deal with the SARS-CoV-2 virus. Drugs such as interferon, remedesiver, and hydroxychloroquine have been tested as potential treatment options. However, only remedesiver has received approval so far.
Fenofibrate is a commonly prescribed oral drug for the management of high levels of fatty substances in the blood such as cholesterol and lipids. Importantly, the drug is approved for use in several countries and has approval from prominent regulatory bodies such as the National Institute for Health and Care Excellence (NICE) and the US Food and Drug Administration (FDA).
"The development of new more infectious SARS-CoV-2 variants has resulted in a rapid expansion in infection rates and deaths in several countries around the world, especially the UK, US and Europe. Whilst vaccine programmes will hopefully reduce infection rates and virus spread in the longer term, there is still an urgent need to expand our arsenal of drugs to treat SARS-CoV-2-positive patients," stated Dr. Farhat Khanim, corresponding author of the study.
Reducing Infection Significantly
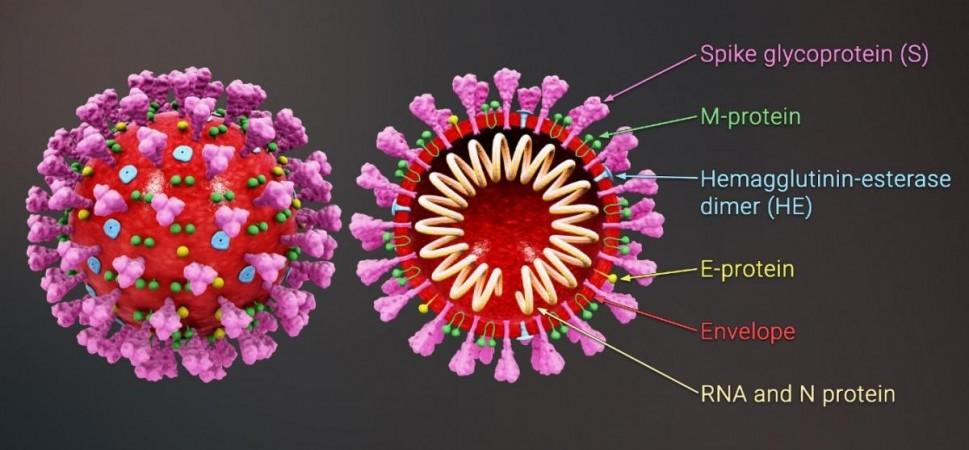
The SARS-CoV-2 virus infects cells using its 'spike' or 'S-protein', a hook-like protein structure found on the surface of the viral envelope. Its prime target is the ACE2 receptor—an enzyme present on the surface of certain cell lines—with which it binds to invade cells. For the study, the authors tested an array of approved drugs, including fenofibrate, in order to identify an ideal candidate that can disrupt the interaction between the SARS-CoV-2's spike and the ACE2 receptor.
After identifying fenofibrate as a viable candidate, the efficiency of the drug in decreasing infection in cells was evaluated. The original strain of SARS-CoV-2 that was isolated in 2020 was used to infect cells in the laboratory. Promisingly, fenofibrate was found to reduce infection by up to 70 percent.
Another important finding was that fenofibric acid/fenofibrate was able to inhibit the binding between the ACE2 and the RBD (receptor-binding domain) of the spike. RBD is the region of the spike where the binding between it and the ACE2 takes place. The authors suggested that it is this effect that may, at least in part, be the source of the drug's antiviral activity. "To our knowledge, this is the first experimental evidence that fenofibrate can modulate RBD and ACE2 proteins and inhibit SARS-CoV-2 infection," they wrote.
Testing Efficiency in Hospitalized Patients
While not published in the current study, additional data indicated that the cholesterol drug is equally effectual against two variants of concern (VOC)—Alpha and Beta. The study of fenofibrate's effectiveness against another VOC—Delta variant—and the exact mechanism of the antiviral activity is ongoing. In order to test the drug in hospitalized COVID-19 patients, the scientists are calling for clinical trials.
Based on the data of the study in its pre-print, two clinical trials have already been registered for treating COVID-19 patients who require hospitalization with fenofibrate. One was applied for by the Hospital of the University of Pennsylvania (USA) while the other was sought by the Hebrew University of Jerusalem (Israel). The team believes that using fenofibrate may help mitigate the effect of the pandemic till complete vaccination is achieved across the world.
Though vaccination programmes are progressing at speed, significant proportions of the population are unlikely to be vaccinated until 2022. Further, while vaccination has been shown to reduce infection rates and severity of disease, scientists are not sure of the strength and duration of the response. "Therapies are still urgently needed to manage COVID-19 patients who develop symptoms or require hospitalization," said Dr. Alan Richardson, co-corresponding author of the study.












!['Had denied Housefull franchise as they wanted me to wear a bikini': Tia Bajpai on turning down bold scripts [Exclusive]](https://data1.ibtimes.co.in/en/full/806605/had-denied-housefull-franchise-they-wanted-me-wear-bikini-tia-bajpai-turning-down-bold.png?w=220&h=138)
![Nayanthara and Dhanush ignore each other as they attend wedding amid feud over Nayanthara's Netflix documentary row [Watch]](https://data1.ibtimes.co.in/en/full/806599/nayanthara-dhanush-ignore-each-other-they-attend-wedding-amid-feud-over-nayantharas-netflix.jpg?w=220&h=138)



