The mention of COVID-19 testing is likely to evoke imagery of a technician collecting nasopharyngeal swab (NPS) samples. However, the obtaining of samples in this manner, and the post-collection processing are bound by safety and logistical constraints. Saliva, on the other hand, is easier to collect but considered unreliable for diagnostic accuracy. Now, scientists have found that subjecting saliva to a simple processing step before testing can provide results with higher sensitivity compared to conventional NPS samples.
According to a new study, a novel protocol that processes saliva in a bead mill homogenizer—a device that vigorously spins test tubes containing samples—before an RT-PCR (real-time reverse transcription-polymerase chain reaction) test can provide results with high accuracy. The method can potentially make large-scale testing for the SARS-CoV-2 coronavirus infection easier and eliminate safety risks posed to health care workers.
"Saliva as a sample type for COVID-19 testing was a game-changer in our fight against the pandemic. It helped us with increased compliance from the population for testing along with decreased exposure risk to the healthcare workers during the collection process," said Dr. Ravindra Kolhe, lead investigator of the study, in a statement.
Exploring New Testing Protocols

RT-PCR is considered the gold standard for COVID-19 testing and continues to remain the most reliable diagnostic technique for detecting the SARS-CoV-2's viral proteins in potential patients. However, in spite of its accuracy, it is bound by several practical limitations, particularly when time is of the essence. These include the need for large laboratory infrastructure, training of professionals, costs, and most importantly, long waiting periods for results. Obtaining the results of an RT-PCR test can take anywhere between 24 to 48 hours.
For the study, the authors examined 429 matched sample pairs—NPS and saliva samples—acquired from 344 individuals either in a community setting(a drive-through testing site) or a healthcare setting (a hospital and nursing home). During the first phase of the study using a procedure called protocol U, 240 matched sample pairs were tested for COVID-19 using RT-PCR. In the second phase, using SalivaAll—a clinical validation for sensitive saliva testing—189 matched pairs were analyzed. These samples also included 85 that had already been investigated with protocol U.
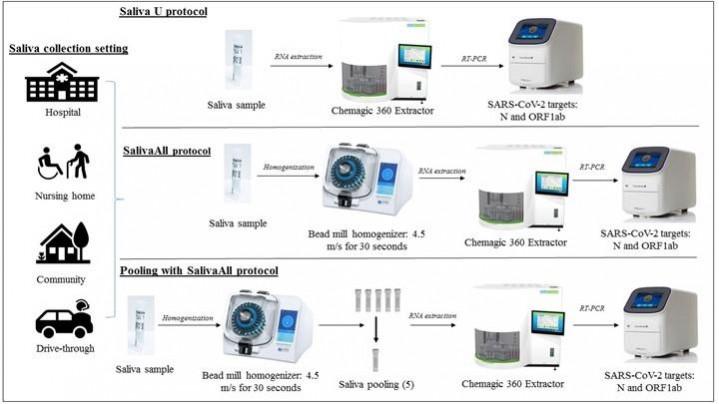
The saliva samples were processed in an Omni bead mill homogenizer prior to RT-PCR testing during the second phase. Next, the authors conducted an additional study using both protocol U and SalivaAll to ascertain whether bead homogenization played a role in the clinical sensitivity of NPS samples. As a final step, the scientists evaluated a five-sample pooling strategy and generated 20 positive pools from 20 saliva samples previously confirmed to be positive for SARS-CoV-2. Each pool contained one positive and four negative samples and was processed with bead homogenizer prior to pooling for COVID-19 RT-PCR testing. They were compared to controls later.
Improved Accuracy of Saliva Samples
In Phase I of the study, 28.3 percent of samples tested positive for the novel coronavirus either from saliva, NPS, or both. As anticipated, the rate of detection was much lower through saliva samples when compared to NPS (50.0 percent vs. 89.7 percent). However, Phase II of the study yielded contrasting results. 50.2 percent of the samples were found to be positive for SARS-CoV-2 either from NPS, saliva, or both. Interestingly, the detection rate in saliva was considerably higher in comparison to NPS (97.8 percent vs. 78.9 percent). Among the 85 samples evaluated with both the protocols, the detection rate was 100 percent for samples tested using SalivaAll. For the samples tested with protocol U, the detection rate was 36.7 percent.

According to Dr. Kolhe, the problems surrounding the lower sensitivity of saliva samples to RT-PCR testing may be a result of their gel-like texture. He highlighted the difficulty faced while attempting to transfer samples accurately into extraction plates. Including a step for homogenization provided saliva samples with uniform consistency and viscosity, which made their pipetting easier for testing. The successful demonstration of the five-sample pooling strategy by the researchers also adds credence to the criticality of pool testing in mass surveillance as daily life takes measured steps towards normalcy.
"Monitoring SARS-CoV-2 will remain a public health need. The use of a non-invasive collection method and easily accessible samples such as saliva will enhance screening and surveillance activities and bypass the need for sterile swabs, expensive transport media, and exposure risk, and even the need for skilled healthcare workers for sample collection," concluded Dr. Kolhe.

















