The announcement of Coronil, an Ayurvedic cure for coronavirus, gave a shimmer of hope to many Indians amidst the turmoil that is caused by the COVID-19 pandemic. But there was also a fair share of skepticism on the bold claim made by Baba Ramdev, who said the herbal medicine could cure coronavirus in just seven days with 100 percent favourable results.
Patanjali's anti-COVID medication kit turned into a meme-fest for many, but the news instantly made the headlines across all channels. Hours after yoga guru Ramdev came out with a "medicine" for Covid-19, the Centre has asked his Patanjali Ayurved Ltd to stop advertising or publicising its claims till the issue is "duly examined".
Taking cognizance of media reports, the Ayush Ministry said that "facts of the claim and details of the stated scientific study are not known to the Ministry".
It also said that the government has informed the company that such advertisements of drugs including ayurvedic medicines are regulated under the provisions of Drugs and Magic Remedies (Objectionable Advertisements) Act, 1954 and Rules thereunder and the directives issued by the Central Government in the wake of COVID outbreak.
Demanding Coronil case study
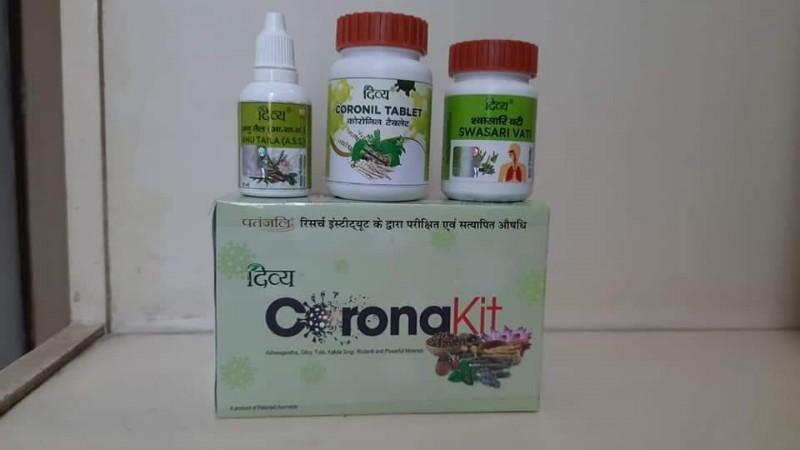
On Tuesday, Ramdev launched Patanjali's Coronil tablet, which he claims is a cure for Covid-19. Patanjali claims those administered the medicine were fully cured and none died. Ramdev even said that 69 percent of them recovered within 3 days.
The Ayush Ministry also reminded Patanjali of a gazette notification, issued on April 21, asserting what are the requirements and manner of research studies on Covid-19.

A Ministry statement said: "In order to make this Ministry aware of the facts of the aforesaid news and verify the claims, Patanjali Ayurved Ltd has been asked to provide at the earliest details of the name and composition of the medicines being claimed for COVID treatment; site(s)/hospital(s), where the research study was conducted for Covid-19; protocol, sample size, Institutional Ethics Committee clearance, CTRI registration and results data of the study (ies) and stop advertising/publicizing such claims till the issue is duly examined."
Meanwhile, the Ministry has also asked the Uttarakhand government's licensing authority to provide copies of license and product approval details of the ayurvedic medicines which the yoga guru claimed, treats Covid-19, a global pandemic that has the world worried.
A miscommunication, says Ramdev

In response to Ayush ministry's statement, Ramdev said all necessary permissions have been taken right from the beginning and the information pertaining to the drug has been sent to the ministry.
"We are not doing any promotion or advertisements based on false claims. If we conducted a clinical trial and found that 100 per cent of patients recovered, will we not tell that to people? Why will we lie?," Ramdev said, according to IndiaToday
Ramdev dismissed claims of violations baseless and "nonsense," reminding that there is no scope for controversy. The herbal medicine firm has acquired approvals from the CTRI and ethics committee and the study was conducted along with NIMS Jaipur.
"The Ayush Ministry should also be patient and support our good work. If we had made false claims without conducting controlled clinical trials on purely myths or hearsay, they should reprimand us. But now that our clinical study has shown effective results, the government should also express pride. We have spoken to Ayush officials, they said we don't have information on the drug, so we sent it to them," Ramdev said.
(Some inputs from IANS)

















