The top 300 widely used drug brands will have the quick response (QR) codes to ensure authenticity and traceability of the medicines, as per the Union Health Ministry which has amended the Drugs Rules, 1945, to implement the innovative scheme.
In March, the ministry had asked the department of pharmaceuticals (DoP) to shortlist drug brands that can be included in the implementation of mandatory QR codes.
The National Pharmaceutical Pricing Authority (NPPA) has identified the 300 most used drugs for QR codes including analgesics, vitamins, and diabetic, and hypertension medicines, among others.
The industry has welcomed the steps of introducing QR codes on widely used medicines which will help in preventing drug counterfeiting which has emerged as a threat in recent years and also will help the common citizens to gather accurate information about the medicines they are using.
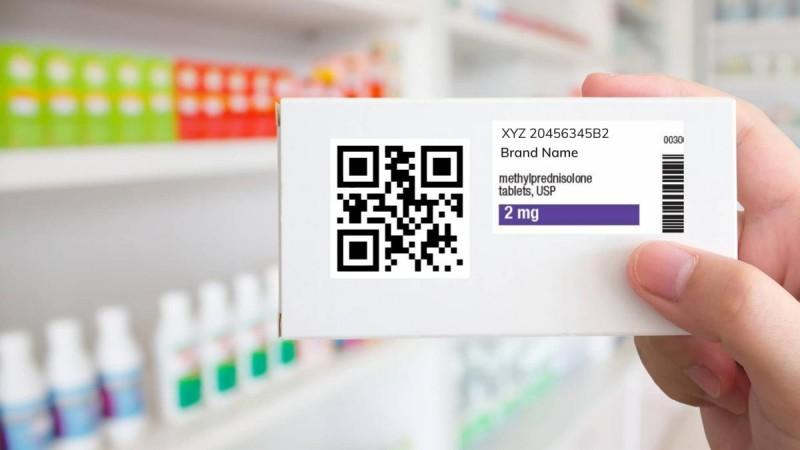
"We welcome the government's move to start only with the top 300 brands as it will allow the ecosystem to be built up before expanding across the sector", said Nishant Berlia, Chairman, Pharmaceutical Manufacturing Committee, PHDCCI. However, he also underlined some challenges to the implementation of the scheme.
Challenges galore
There are three challenges, he explained. The first is implementing QR printing is a significant change control in the manufacturing process and requires time to deploy. Second, there will be cost escalation for small and medium manufacturers who make lower-priced but small batches and will conversely have a higher cost to implement per batch.
"Thirdly, the availability and affordability of IT systems at last-mile distribution in our country mean that many counters most at risk will take time to participate without subsidies or grants", said the Chairman of Pharmaceutical Manufacturing Committee.
"Drug counterfeit has recently emerged as a global threat and the QR Codes for drug safety can go a very long way in managing drug counterfeit and proper administration of medicines to patients.
Medicine packages that now come with QR Codes offer transparency about the manufacturing process, contents of the drugs and expiry date", said Nikkhil K Masurkar, Executive Director, ENTOD Pharmaceuticals. He added that QR codes can even allow for better data monitoring by a pharmaceutical company.
"It is certainly a welcome move by the government which will soon make QR code mandatory on the pack of 300 medicines. The medicine packs that come with a unique QR code, will help trace the source and affirm the authenticity of products," he added.

















