A recent study on how a gene discovered in monkeys and mice may act as a new form of antiviral to protect people against HIV, Ebola, and other deadly viruses were released online and will be published in the journal Cell on October 14. This discovery may indicate a novel form of immunity that may develop rapidly to defend against short-term health hazards.
The team led by scientists from the University of Utah Health and the Rockefeller University conducted the investigation. The study shows that the retroCHMP3 gene, which is found in mice and other mammals, has the potential to block some viruses from exiting an infected cell and infecting additional cells.
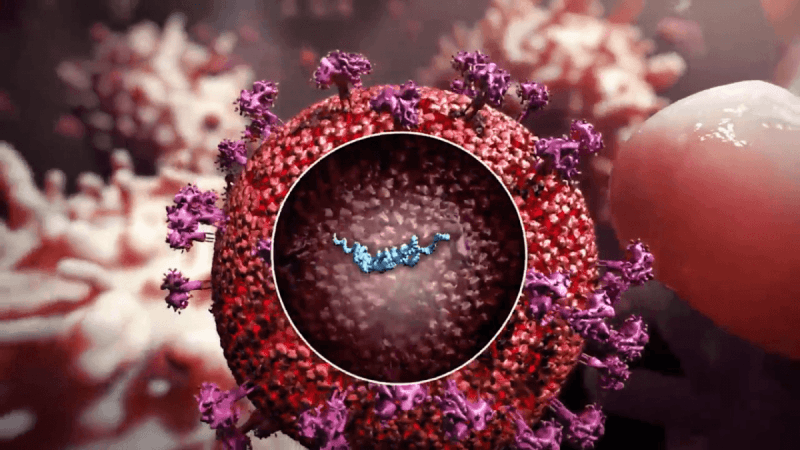
The human body contains a gene known as charged multivesicular body protein 3 (CHMP3). RetroCHMP3 is a duplication of this gene. HIV and several other viruses exploit the CHMP3 pathway to bud out from the cell membranes and infect additional cells.
Their research suggested that the duplications of CHMP3 seen in primates and mice served as a protective mechanism against viruses like HIV and other viral infections. Nels Elde, senior author of the study and an evolutionary geneticist in the Department of Human Genetics at U of U Health and his colleagues intended to test if retroCHMP3 versions worked as antivirals.
Other labs have effectively stopped HIV from budding off cells using a shorter, tweaked form of human CHMP3. However, there was a flaw -- the changed protein also affected critical cellular processes, resulting in cell death.
Elde and his U of U Health colleagues attempted something new with scientists Sanford Simon from Rockefeller University, Phuong Tieu Schmitt and Anthony Schmitt from Penn State University. They used genetic techniques to get human cells to make the squirrel monkey's version of retroCHMP3. Then scientists treated the cells with HIV, which had trouble budding off the cells, thus halting them. All without affecting metabolism or other biological activities that might trigger cell death.
Wes Sundquist, the co-corresponding author of the research and chair of the Department of Biochemistry at the University of Utah, believes the finding is exciting. "We always assumed that cells could protect itself against viruses at this stage, but we couldn't understand how that might occur without trying to interfere with other critical cellular functions."
Previous studies done
A study published in Current Medicine Research and Practice examined whether gene editing might be used to develop an HIV cure.
Clustered CRISPR and the Cas9 technology aim to cure rather than suppress HIV. CRISPR-Cas9 uses a guide RNA that complements CCR-5, a gene found on white blood cells. The Cas9 nuclease detects the two binders. Cas9 acts like a scissor, cutting the target complex and inactivating it. Current CRISPR-Cas 9 strategies target the CCR-5 gene to impart HIV resistance.
Another study was conducted to see if the in vivo production of monoclonal antibodies through electroporation defends against lethal influenza and Ebola infections.
For the manufacture of functional mAbs in vivo, the scientists present an improved electroporation-based DNA gene transfer platform technology, which might minimize costs and administrative constraints. They showed that by utilizing DNA dosages and therapeutically practical electroporation settings, several mAbs may be produced at protective concentrations for extended periods at high levels.
Additionally, the expressed mAbs may protect mice against fatal influenza or Ebola virus infections. As a result of the findings, this DNA gene transfer platform technology may revolutionize worldwide mAb therapeutic availability.

















