
It's a theme worth a ponder for the sake of thy health – medicine prices. The government has been doing that for quite a while, though what has not come out of the exercise is cheaper prices for vital life-saving or malady-busting drugs. Balancing the interests of patients and the industry is a tight-rope walk and negative implications can rise when done without policy finesse.
Like the shopping list arbitrarily imposed to artificially drive down prices of medical and equipment like artificial knee joints and cardiac stints well below their manufacturing costs, the Draft Pharmaceutical Policy 2017 prepared by the Department of Pharmaceuticals largely typifies a bureaucracy-driven law which is high on control and middling on potential results for the intended beneficiaries. The positives and negatives are there to see in the draft released a few weeks back, and a few pleasant surprises this document contains have been long in the making.
There is much in the policy to cheer about; and much to be worried. One of the more pleasant ones is the government almost admitting the complicity of Indian pharmaceutical companies in fabricating results of clinical trials and spurious quality of certain categories of manufactured drugs.
The draft policy has devoted an entire paragraph to quality concerns affecting the domestic pharmaceutical market. The GVK Bio clinical trials scam is fresh in public memory, and apparently, government memory as well: a lesson strong enough for the Ministry of Health to take industry lobby Indian Pharmaceutical Association's (IPA) mea culpa on clinical trials being fabricated seriously and decide to sharpen the end-game of its policy reform.
Bringing drug manufacture processes under the World Health Organisation's (WHO) umbrella will be quite a reform towards speeding up quality assurance, though a better direction would have been to address the deficit of specialised staff. The Drug Controller General of India faces a huge shortage of inspectors which will be a problem when it comes to providing the right amount of oversight and pharma compliance with WHO processes.
The unequal prescription
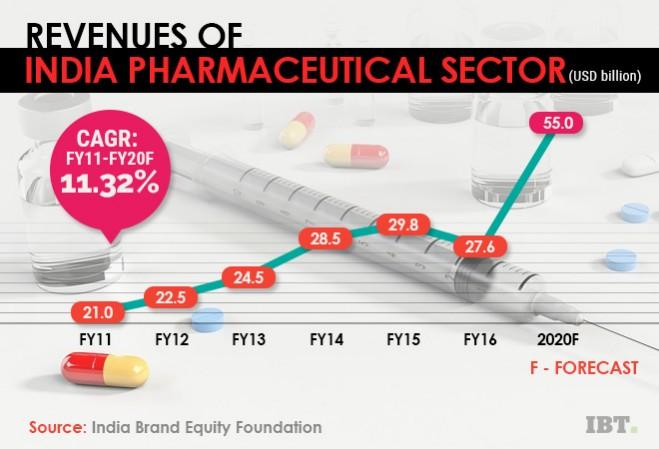
The draft pharmaceutical policy reflects years of public and regulator worries on the directions taken by one of India's most slowdown-resistant industries. As things stand, it took years of appeals and sanctions imposed by European and American regulators for the IPA to admit that 85 per cent of the drug supply in India has no proven therapeutic efficacy or safety.
Contrarily, the draft pharmaceutical policy does confound things to the extent of actually specifying the terminology which medical practitioners should use while writing out their prescriptions. The draft also reiterates the government's desire for prescriptions to be written for generic drugs rather than branded drugs.
The physician-to-pharmacy cycle stands to be disrupted by the draft pharma policy proposal, not least because of the arbitrary price controls being planned, as due to confusion likely to arise if chemical (or pharmacopeial) names are preferred over the more well known brand names while cutting out a prescription for the patient. For example, a patient suffering from high fever who goes to the doctor, is often prescribed Crocin. However, as per the new regulation, the doctor will not be allowed to mention the brand name 'Crocin', and would instead have to prescribe the chemical name of the drug. The balance of power when it comes to brand promotion could very well shift from doctors to chemists if such a policy proposal were to be adopted.
The greater danger of this move is that it could kill off brand differentiation and erode the value of high-end medical products, which can disrupt the industry's value chain in the short to medium term.
The lack of effective control of chemists is something the draft policy fails to address. Most of them do not ask for prescriptions when selling schedule drugs, thanks to which fake medicine makers mint easy money. The presence of large drug store chains could streamline the distribution system and inject quality consciousness into the sales process.
Bringing up a positive angle of the policy assault is the National List of Essential Medicines (NLEM). The new draft policy is expected to eventually take control over NLEM and could have sweeping powers to decide drug prices and alter or prioritise specific drugs on the list for price monitoring. The policy claims to look at "moving from price control to monitoring of drug prices, their availability and accessibility". This move will have strong positives in pushing the envelope on consumer choice and ensuring that quality and innovation stay on top of the pharma industry's radar.
Nevertheless, a huge setback from the draft policy, if translated into action, could be for contract manufacturing companies engaged in third party manufacturing, which has created lakhs of skilled and semi-skilled jobs in India's major pharma hubs.

Policy perils for the third empire
Some of the steps outlined in the draft policy such as eliminating third party manufacturing and loan licencing would hit the industry as well as the availability of medicines.
Under third party manufacturing, companies are permitted to manufacture products at another company's plant and then market them under their names. The need for such a move is unclear at best and precipitous at worst, as it is tailor-made to bring India's giant pharmaceutical hubs to their knees, and push third party manufacturers out of business.
India's Tier I cities are growing at approximate 10 percent per annum when it comes to contribution of total sales of the pharmaceutical industry. Rural areas are growing at about 14.5 percent per annum, and growth in these areas would be hit by a negative policy headwind, as they are predominantly hubs for third party manufacturers.
A location most affected by such a move would be Baddi in Himachal Pradesh's Solan district, with an annual turnover of Rs 30,000 crore, of which Rs 9,500 crore involves exports. Baddi is globally ranked as the third biggest pharmaceutical hub, manufacturing more than 150 bulk drugs, with demand coming in from 200 countries.
Sub-contracting has been considered a feasible system to efficiently bring in smaller units under the quality control of large companies.
The Indian pharmaceutical industry, though fairly fragmented with a good chunk of the unorganised sector falling within its rarefied environs, still has the top 10 companies contributing 41 percent of total sales. The next ten companies account for 22 percent of sales while the remainder contributes 37 percent. Urban regions (metros and tier I cities) fetch about 60 percent of total sales while the rest of the country contributes the balance 40 percent.
One positive from the draft policy is stricter checks and audits of drug manufacturing plants to ensure better quality. While large pharma companies already adhere to higher standards due to their international market exposure, fly-by-night operators would find it difficult to operate in such an environment. Large pharma companies with well-established manufacturing plants would thus be in a good position to gain market share.

Selling medicines under their pharmacopeial names could intensify the price war in the pharma market which may not benefit patients and manufacturers. The patented drug market virtually survives on brand names, and forcing them to sell under pharmacopeial names would make manufacturers unduly dependent on FDCs to maintain their brands.
The Sun Pharmas and the Ranbaxys depend on wider points of sale and huge distribution networks, besides economies of scale yielded by multiple manufacturing facilities. But the cutting-edge ones like a Cadila, a Mankind Pharma, or a Natco Pharma live or die on the strength of their drug portfolio. The draft policy should look at proactively accommodating their distribution and manufacturing concerns as well.

















