"We don't have kits at all," said a professional from one of the accredited private diagnostics firms. "The government labs do not have the supplies either," he reiterated.
Weeks into the coronavirus outbreak and the nationwide imposed lockdown, the frontline officers combatting Covid-19 - the healthcare professionals, nursing staff and doctors lack coronavirus testing kits to detect infected patients.

According to findings from the Indian Council of Medical Research, "123 state-run labs are operating at only 36% capacity, while the 49 accredited private labs manage just an average of eight tests each on Monday," a news report stated.
Shortage in supply of coronavirus testing kits across India
While the nation's healthcare system is struggling to procure imported coronavirus testing kits in demand, for accurate and speedy testing, the red-tapism in the country is impeding deployment efforts of locally-manufactured COVID-19 testing kits. International manufacturers prioritise the supply of coronavirus testing kits to countries with alarming incidences of Covid-19 such as Spain, Italy, the US, and the UK. India ranks perhaps the last since the pandemic is slowly taking shape and form in the nation, and most international vendors are not sure of their testing kit allocations to India, amidst the global coronavirus outbreak.
With an official tally of just more than 2,000 Covid-19 cases across the nation, and not making most of the lockdown to curb and control the spread of the pandemic, could mean underestimating the virus's potential to spread fast in densely packed cities. With meager public finances and an ill-equipped healthcare system, the nation is being overexposed to such fast-spreading contagion could spell disastrous times calling.
Shutting down of state borders and international import ban has made it extremely difficult for India to procure certain chemicals from foreign nations to assess a patient's sample. While the nation is far ahead of the global counterparts in manufacturing generic drugs, the biopharmaceutical industry's capability to manufacture higher-end medication and devices is crippled. India still lacks the availability of the virus-testing kits that identify the pathogen's genetic sequence in an infected patient's sample. With increasing demand and shortage in supply, the testing kits available in the market today are most often overpriced.
Is local manufacturing the answer to testing kit availability woes?
The transport system is halted and India is facing a bottleneck in foreign imports leading to a huge shortage of coronavirus testing kits. The Indian government is giving the positive nod for testing kits manufactured in the US and Europe, approved by the International regulatory bodies for imports into the country. The local manufacturing environment is heavily plagued by red-tapism and many formal regulatory approval processes.

The Indian government further has appointed only one government facility in the city of Pune for the regulatory approval process, which means any manufacturer outside the city is finding it difficult to procure approvals amidst the nationwide lockdown.
Mylab Discovery, a Pune-based startup is the only local manufacturer approved at the moment with a capacity to produce 20,000 tests a day. In order to scale up the operations and escalate faster delivery of coronavirus testing kits, the Mylab Discovery Solutions has partnered with Serum India's CEO Adar Poonawalla and Abhijit Pawar, Chairman of APG. The investments will be used for scaling the production of COVID-19 testing kits and the expansion of molecular diagnostic solutions. It is the first-of-a-kind Covid-19 testing kit, manufactured in India to have received commercial approval from Indian FDA, Central Drugs Standard Control Organisation (CDSCO). It is named as Mylab PathoDetect COVID-19 Qualitative PCR kit.
What's important in these testing times is the affordability of the testing kits produced or procured, as importing testing kits from abroad can be quite an expensive proposition. Chennai-based manufacturer of medical devices, Trivitron Healthcare though is not able to reach Pune but is hopeful of receiving approval for its testing kits that can produce results, at an affordable cost of Rs 500 ($6.50) per patient.
The US can afford to pay much more for the procurement of coronavirus testing kits, but for India, it's of utmost importance to ensure low-cost procurement, manufacturing and easy availability of these testing kits to meet affordability constraints of the huge population.
Rapid antibody testing can help speedy detection of Covid-19: ICMR
While the issue of manufacturing testing kits and procurement continues to be a challenge, the Indian Council of Medical Research has come up with a recommendation to conduct rapid antibody blood testing at various coronavirus hotspots across the nation. This could help in speedy and early detection of coronavirus infected individuals.
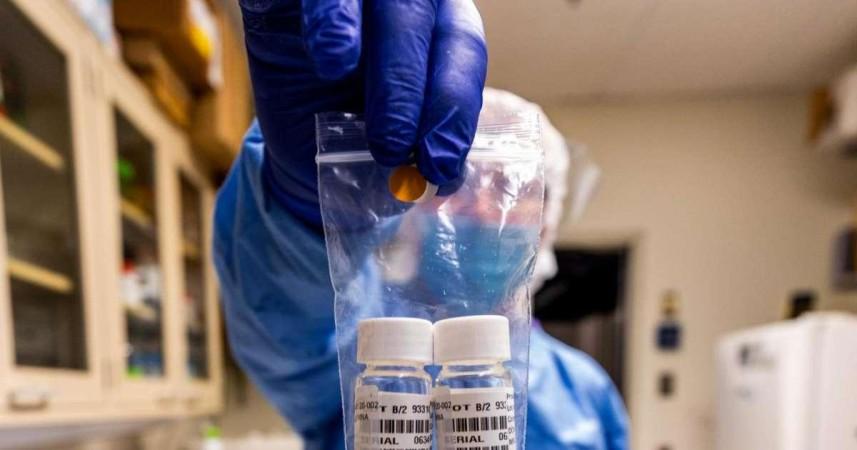
The recommendation decision was taken during an emergency meeting of the National Task Force formed to deal with the coronavirus situation. "Population in hotspot areas may be tested using rapid antibody tests. Antibody positives to be confirmed by RT-PCR (reverse transcription-PCR) using throat/nasal swab, and antibody negatives will be quarantined at home," the ICMR said in its interim advisory.
The Ministry of Health has identified 22 potential and 20 existing Covid-19 hotspots across the country for rapid antibody testing. The antibody tests are similar to other blood tests, however, the results in the case of Covid-19 infection found in patients are available in 15 to 30 minutes.
At present, the government is using the PCR (polymerase chain reaction) tests to detect coronavirus from throat samples or nasal swabs of patients with symptoms, and those with international travel history (high-risk individuals) who might have come in contact with coronavirus positive patients.
















