Researchers have developed a novel hydrogel drug delivery system that transforms daily or weekly injections of diabetes and weight control drugs like Ozempic, Mounjaro, Trulicity, Victoza, and others to just once every four months.
While the drugs all work by mimicking the hormone glucagon-like peptide 1 (GLP-1) and help people manage their diets and their weight, the typical daily or weekly injections are a burden for many patients.
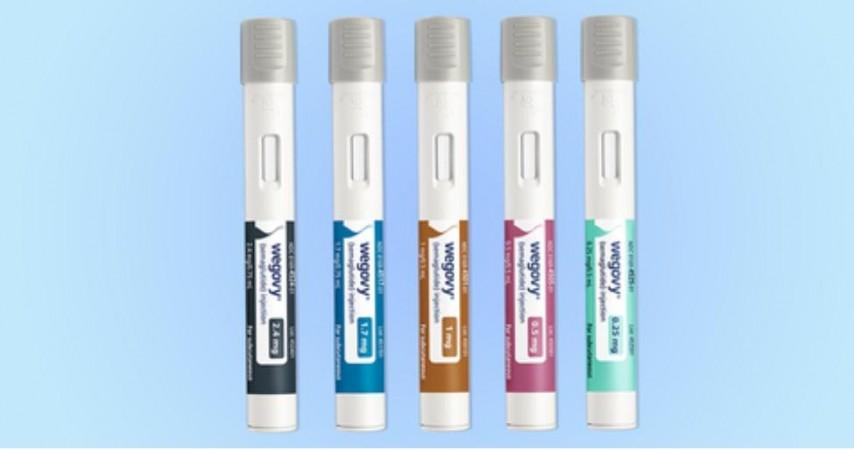
The new hydrogel system allows the slow release of the diet control drugs over many months, which researchers believe will greatly improve management of both diabetes and weight, improve patient drug compliance, and help those with Type 2 diabetes improve long-term health outcomes.
"Adherence is one of the biggest challenges in Type 2 diabetes management," said Eric Appel, Associate Professor of materials science and engineering at Stanford University and principal investigator.
"Needing only three shots a year would make it much easier for people with diabetes or obesity to stick with their drug regimens."
The hydrogel, described in the journal Cell Reports Medicine, has the unique physical characteristics of the nanoparticles at its heart. Hydrogels are not new -- many people today wear contact lenses made of hydrogels, for instance -- but these are engineered to resist tearing and to hold their shape.
Appel's hydrogel is instead engineered with polymers and nanoparticles that are weakly bound to one another, so as to hold together as a gel yet dissipate slowly over time. The hydrogel is formed from a mesh of polymer chains and nanoparticles that hold the drug molecules until the mesh dissolves away, releasing the drugs.
"Our hydrogel melts away over many months like a sugar cube dissolving in water, molecule by molecule," Appel explained.
So far, the team has tested the new drug delivery system in laboratory rats with high success. In rats, a single injection of this hydrogel-based therapy improves management of blood glucose and weight compared to daily injections of a leading commercial drug, Appel noted.
Next up will be tests in pigs, whose skin and endocrine systems are most similar to those in humans. If those trials go according to plan, Appel could see human clinical trials within a year and a half to two years.
(With inputs from IANS)















