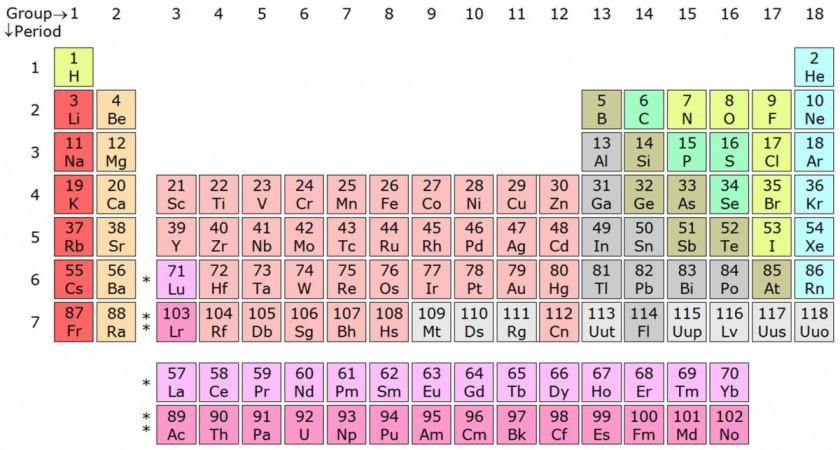
If one's paid attention to their high-school chemistry lessons, then they'd know that elements are grouped according to their atomic numbers on the periodic table of elements and that the bottom rows are where the man-made elements are placed. Now, the latest reports suggest that the new textbooks will need to add four more elements to the list. The elements are nihonium (Nh), moscovium (Mc), tennessine (Ts) and oganesson (Og).
The four man-made elements, which were so far recognised by only their atomic numbers 113, 115, 117 and 118, have been recognised by the International Union of Pure and Applied Chemistry, or IUPAC.
The four elements' names follow the tradition of naming elements after key figures in science or a geographical location. However, the names, like any new employee at their job, are on probation.
IUPAC will be allowing members of the public to review the names assigned to these seventh-row elements. It also invites members of the public to share their two cents here.
Of the four elements that received names, element 113 or nihonium was created in Japan, CNN reported, and it was named after "Nihon," one of the two ways to say "Japan." It is also the first element to be created in Asia. Elements 115, 117, created by two Russian-American teams, have been named moscovium and tennessine, respectively, after the cities in which they were discovered — Moscow and Tennessee. Element 118 or oganesson was also created by a Russian-American team and named the element after Yuri Oganessian, a pioneer in superheavy elemental research.
IUPAC's guidelines state the elements that fall under groups 1 to 16 of the periodic table, which includes alkali metals, alkaline earth metals and metals used to make coins end with the suffix "-ium." Group 18 elements end with "on" like neon, xenon and other noble gases. The suffix "-ine" is attached to elements that form group 17 on the periodic table and includes halogens like chlorine, fluorine and iodine.










