If there is one thing that the ongoing COVID-19 has brought to attention, it is the need to detect pathogens quickly and act on containing their spread. Thus, time is of the essence. However, detection alone does not guarantee an infection or contagiousness. Now, scientists have reported the development of a new sensor that can not only detect the presence of a virus but also help ascertain whether it is infectious.
In a new study, a multi-institutional team of researchers described a new sensor that combines specially designed aptamers (DNA fragments) and nanopore sensing. It can target and identify infectious viruses within minutes and does not require pre-treatment of samples. The efficiency of the sensor was demonstrated using two viruses that are currently affecting millions of people across the world: the SARS-CoV-2 virus and the human adenovirus.
"Applications of the aptamer-nanopore sensors in different types of water samples, saliva, and serum are demonstrated for both enveloped and nonenveloped viruses, making the sensor generally applicable for detecting these and other emerging viruses of environmental and public health concern," said the study. The research was published in the journal Science Advances.
Contradiction of Viral RNA Levels

Currently, the 'gold standard' for viral detection—including SARS-CoV-2—is PCR (Polymerase chain reaction) tests. While they can detect viral genetic material, PCR tests cannot discern whether the sample is infectious or if the person to whom it belongs is contagious. According to the authors, this can complicate the process of tracking and containing viral outbreaks.
Dr. Yi Lu, co-lead author of the study, emphasized that this specific aspect plays a crucial role in COVID-19. The level of viral RNA has been found to have a minimal association with SARS-CoV-2's infectivity. During the early stage of the COVID-19 infection, the amount of viral RNA in an infected person is low and its detection is difficult. However, the individual is highly contagious.
"When a person is recovered and not infectious, the viral RNA level can be very high. Antigen tests follow a similar pattern, though even later than viral RNA. Therefore, viral RNA and antigen tests are both poor in informing whether a virus is infectious or not. It may result in delayed treatment or quarantine, or premature release of those who may still be contagious," highlighed Dr. Lu.
Need for Rapid Detection and Differentiation

Tests known as plaque essays, which aid in the detection of viruses, exist. However, they require specific preparation and at least a few days of incubation to yield results. PCR tests are also bound by certain practical limitations. These include costs, training of professionals, the need for large laboratory infrastructure, and most importantly, lengthy waiting time for results. Results of an RT-PCR test can take anywhere between 24 to 48 hours to be obtained.
Another important aspect of the detection of viruses is being able to distinguish infectious ones from non-infectious ones. Furthermore, being able to discern small quantities from untreated samples that may also contain other contaminants is essential. This is important not just for the quick diagnosis among individuals in the early stages of an infection or if they are still contagious following treatment but also for environmental monitoring, noted Dr. Benito Marinas, co-lead author of the study.
Distinguishing Between Viruses
According to the authors, the new sensor-based method addresses the abovementioned vital facets of viral testing. They reported that the sensor can provide results in 30 minutes to two hours. Also, as the samples do not require pre-treatment, the test can be used to identify viruses that will not grow in labs.
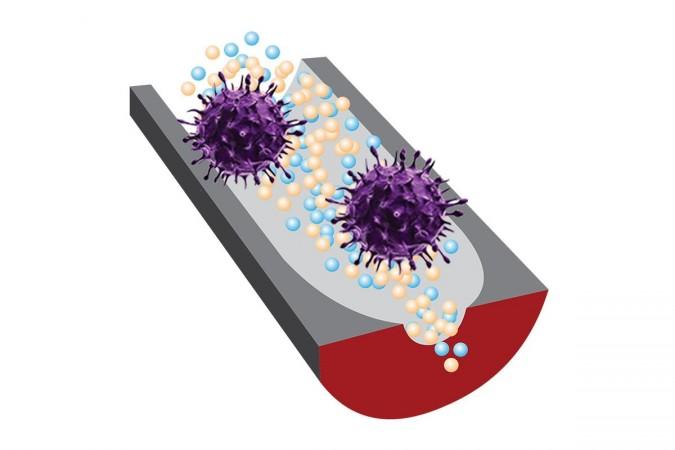
Describing the new detection technique, Dr. Ana Peinetti, first author of the study, said, "Our sensor combines two key components: highly specific DNA molecules and highly sensitive nanopore technology. We developed these highly specific DNA molecules, named aptamers, that not only recognize viruses but also can differentiate the infectivity status of the virus."
The DNA aptamers are chosen from a DNA library to bind with intact infectious viruses—but not noninfectious ones—and then integrated into a solid-state nanopore. This permits strong confinement of the virus to increase sensitivity. In order to demonstrate the effectiveness of the sensor, the researchers turned to human adenovirus and SARS-CoV-2. The sensitivity was streamlined down to 1 pfu/ml for human adenovirus and 1 × 104 copies/ml for SARS-CoV-2.
Dr. Marinas stated, "We chose human adenovirus to demonstrate our sensor because it is an emerging waterborne viral pathogen of concern in the United States and throughout the world. The capability to detect infectious adenovirus in the presence of viruses rendered noninfectious by water disinfectants, and other potentially interfering background substances in wastewaters and contaminated natural waters, provides an unprecedented novel approach."
Potential for Scalability, Ease of Use
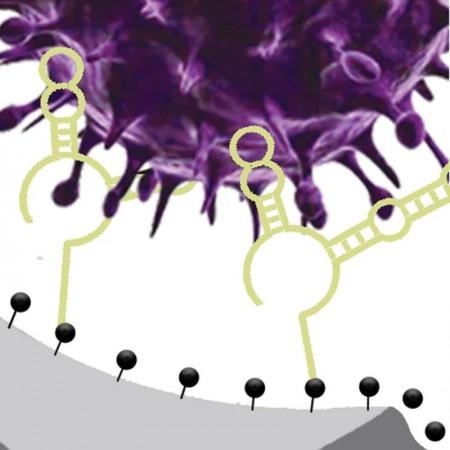
The new sensing technique offers easy adaptability to other viruses. According to the scientists, it can be applied to other viruses by simply modifying the DNA to target different viruses. Additionally, the DNA aptamers utilized in the new sensor can be easily manufactured using existing DNA synthesizers, much like how RNA probes are produced for PCR tests. Dr. Lu highlighted that the commercial availability of nanopore sensors makes the scalability of the sensing method easier.
So what is the next step in the research? The authors have commenced work on improving the sensitivity and selectivity of the sensor. They are working on incorporating their DNA aptamers with other detection methods. This includes features such as sensors to work with smartphones or color-changing dipsticks, which could eliminate the requirement of specialized equipment.
The researchers also expressed hope that through the ability to differentiate between infectious and non-infectious viruses, their technology may also help in unraveling more about infection mechanisms. Dr. Marinas averred that it could also in providing better protection of public and environmental health.
"In addition, the aptamer technology could be further developed into multichannel platforms for detecting other emerging waterborne viral pathogens of public and environmental health concern, such as norovirus and enteroviruses, or for variants of the virus that causes COVID-19," concluded Dr. Marinas.














