When it comes to COVID-19, time is of the essence. The faster a potentially infected person is tested for the SARS-CoV-2 virus-caused disease, the quicker containment measures, and therapeutic interventions can commence. Providing a new screening method, scientists have reported the development of a breathalyzer that can detect the infection in exhaled breath within 15 seconds.
In a new multi-institutional study, researchers have described a new unique breath test that can detect a distinct 'breath print' in patients critically ill with COVID-19. It was found that the test was able to identify the respiratory infection in severely ill patients with high accuracy and speed. The findings of the study were published in the journal PLOS ONE.
"The gold standard for diagnosis of COVID-19 is a PCR test that requires an uncomfortable nasal swab and time in a lab to process the sample and obtain the results. The breathalyzer test used in our study can detect COVID-19 within seconds," said Dr. Matthew Exline, lead author of the study, in a statement.
Need for an Alternative
Currently, the PCR test is the most reliable diagnostic method for detecting the SARS-CoV-2 virus' viral proteins. While it is highly reliable and accurate, certain practical limitations diminish its viability. These include costs, training of professionals, the need for large laboratory infrastructure, and most importantly, lengthy waiting time for results. Results of PCR tests can take anywhere between 24 to 48 hours to be obtained.
Previous researches on respiratory viral infections have demonstrated that the inflammation of the airways leads to the release of inflammatory cytokines. In turn, these cytokines result in the production and release of volatile organic compounds (VOC), ammonia (NH4), and nitric oxide (NO). This process serves as the underlying principle of the breath detecting device's functioning where it seeks a distinct 'breath print'.
"This novel breathalyzer technology uses nanosensors to identify and measure specific biomarkers in the breath. This is the first study to demonstrate the use of a nanosensor breathalyzer system to detect a viral infection from exhaled breath prints," stated Dr. Pelagia-Irene Gouma, corresponding author of the study.
Highly Accurate and Fast
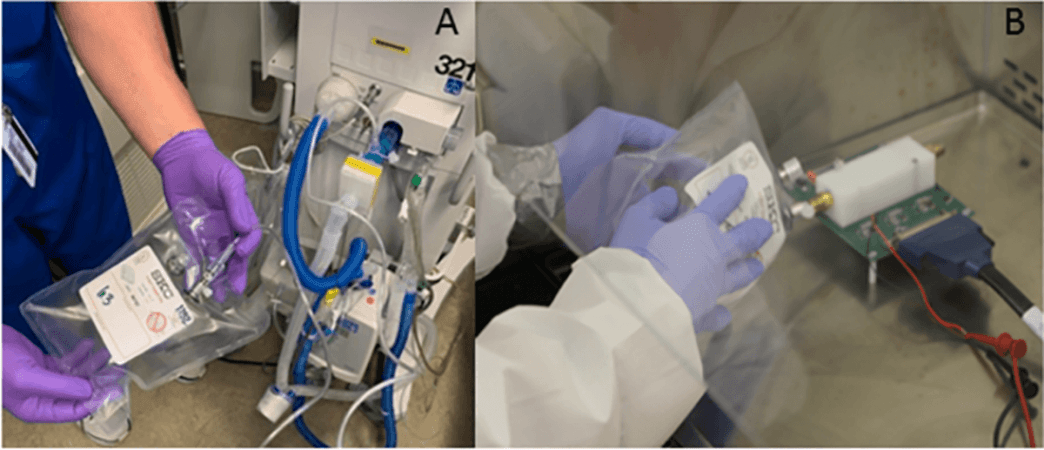
For the study, the team evaluated samples from 46 hospitalized patients who were in the intensive care unit. These patients suffered from acute respiratory failure and required mechanical ventilation. While 23 patients had COVID-19, 23 controls did not. All the patients had undergone PCR tests upon addition to the ICU.
The researchers obtained exhaled breath bags from all the patients on days 1, 3, 7, and 10 of their inpatient stay, or until the end of reliance on mechanical ventilation. Within 4 hours of collection of the breath bag samples, they were examined in a lab using the novel breathalyzer. The data from seven participants was not included as the authors failed to detect the end-of-the-life of the sensor on time. Thus, data from 39 patients were used for the analysis.
It was found that the 'breath print' was identified in patients with COVID-19 pneumonia with an accuracy of 88 percent upon their admission. Importantly, the detection occurred within 15 seconds.
Potential for Rapid Diagnosis
While PCR tests can detect viral genetic material, they cannot distinguish whether the sample is infectious or if the person to whom it belongs is contagious. This can complicate the process of tracking and containing viral outbreaks. Therefore, the utilization of the new breathalyzer technology can aid in the rapid diagnosis and screening in both symptomatic and asymptomatic individuals.

Explaining the advantages of the novel technique, Dr. Exline said, "PCR tests often miss early COVID-19 infections and results can be positive after the infection has resolved. However, this noninvasive breath test technology can pick up early COVID-19 infection within 72 hours of the onset of respiratory failure, allowing us to rapidly screen patients in a single step and exclude those without COVID-19 on mechanical ventilation."
However, the team admitted that the study was not without limitations. All the evaluated patients were critically ill. It is likely that they represented the most acute cases of COVID-19 pneumonia. Also, all the patients were screened for the SARS-CoV-2 virus but not other viruses.
"It is possible, that other coronaviruses will have a similar signal. Due to safety concerns around aerosol-generating procedures, we did not test patients after they were extubated so we were unable to document return to baseline in all patients, but did see in a number of patients as they recovered from COVID-19," wrote the authors.

















