Modern medicine has made strides in reducing maternal deaths during childbirth. With a wide range of treatments and tests available for pre and post-natal care, childbirth has become less risky among those having access to healthcare. However, in spite of medical advancements, there are some natal conditions that still claim maternal lives. Placenta accreta spectrum disorder (PAS) is one such example. Now, a new study has described a novel blood test that can detect the condition in less than 24 hours.
An international team of researchers from multiple disciplines has developed a new test to detect PAS, a rare yet serious placental disorder. The life-threatening condition occurs when the placenta continues to remain attached to the uterus following childbirth. The test opens new avenues into its identification and timely medical intervention.
"Our study demonstrates a promising noninvasive technology for detecting PAS that does not rely on expensive imaging instruments or expertise making it accessible for a range of point-of-care settings including in low resource areas," said Dr. Margareta Pisarska, co-senior author of the study, in a statement. The research was published in the journal Nature.
A Life-threatening Condition

Though rare, placenta accreta spectrum (PAS) disorder has high maternal complications and mortality rates. Also known as abnormally invasive placenta (AIP), PAS is said to occur when there is no spontaneous detachment of the placenta from the uterus after delivery and is anchored deeply into the uterine wall.
PAS can lead to massive blood loss during the course of labor and delivery, and require blood transfusions and admittance to intensive care to mitigate complications. Often, it causes the demise of the mother. The forcible removal of the placenta is ruled out as it can also result in massive bleeding, which can again be potentially fatal. Cesarean hysterectomy following the removal of the fetus is one of the few ways of dealing with PAS.
Previous history of cesarean delivery is the most common cause of PAS. Other causes include damage to the uterine wall during a prior surgery, advanced age of conception, and IVF (In vitro fertilization), among others. Currently, mothers who have a history of pregnancy complications are reviewed using ultrasound to spot signs of PAS. However, despite having no so such history, some women can still be at risk of PAS. This makes existing techniques inadequate for its screening.
Novel Blood Test
The newly-described test utilizes a technology known as a NanoVelcro Chip. Dr. Yazhen Zhu and Hsian-Rong Tseng, collaborators from UCLA (University of California, Los Angeles), have been developing the chip for over 15 years. It was initially designed to spot tumor cells in the blood samples of individuals suffering from cancer. For the new research, the authors modified the chip so that it could identify placenta cells in blood samples from expecting mothers.
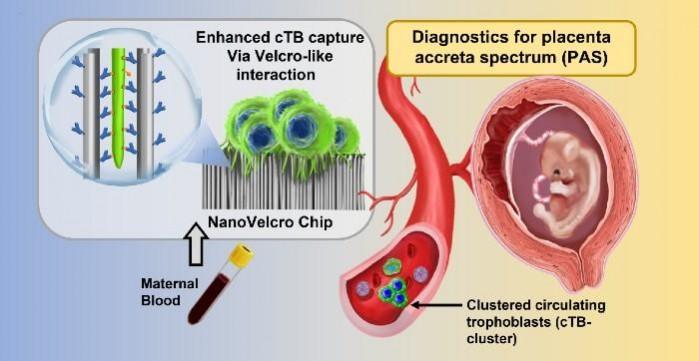
A particular variety of cells known as circulating trophoblasts (cTBs) is detected by the chip. Trophoblasts are involved in the formation of the fetus and placenta. These cells are linked to the emergence of PAS. Not only is the new NanoVelcro test simple but it was also engineered to be implementable within the existing healthcare facilities that provide prenatal care. Only two milliliters of blood are required for the test, which can be performed in the first trimester of pregnancy. This allows for early referrals to doctors who specialize in handling high-risk pregnancies.
Cells are isolated from the collected sample and allowed to incubate overnight on the chip. It captures the number of trophoblasts and trophoblast clusters which are calculated using a fluorescent microscope. If the number of cTBs and cTB clusters are found to be abnormal, it is an indication of a higher risk of PAS. The test and the result can be conducted and obtained in less than 24 hours.
Promising Diagnostic Performance
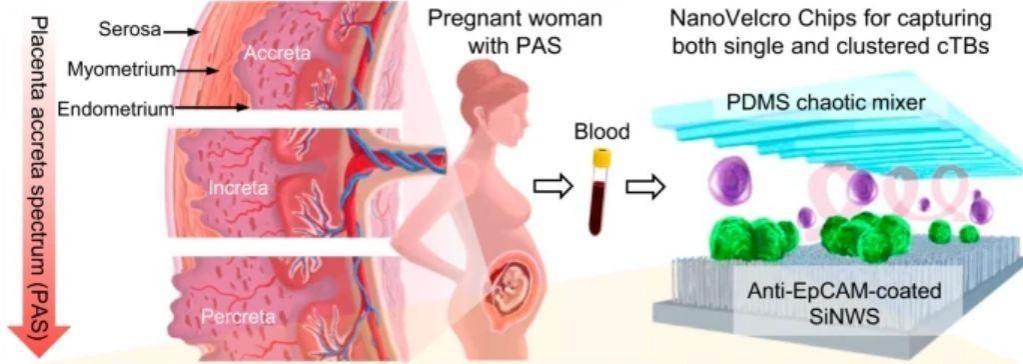
Using the optimized NanoVelcro chip, the team analyzed blood samples from 168 pregnant women and 15 non-pregnant women (control sample). According to its design, the test counted the number of cTBs and cTBs clusters in the examined samples. The test was found to have an 83.8 percent probability of establishing the existence of placenta accreta, and 92 percent chances of rejecting its presence with a negative result.
"Our feasibility study on the enumeration of cTBs and cTB-clusters from 168 pregnant women demonstrates excellent diagnostic performance for distinguishing PAS from non-PAS," wrote the authors in the study. Dr. Pisarska noted that recent studies have revealed a lack of diagnoses of PAS disorder (undiagnosed) in around 50 to 66 percent of the cases before delivery.
Therefore, there is an essential need for the development of new technologies in this regard, she stressed. The team acknowledged that the successful outcome of the research was dependant on the multidisciplinary approach towards it. Currently, the scientists are working on refining the test to enhance its reliability and accuracy.

















