As rapid climate change threatens the future of the planet, clean and sustainable sources of energy are the need of the hour. Luckily, solar energy, whose cost of harvesting has seen a drastic decline in recent years, has emerged as a viable candidate. However, storage has been its primary drawback. Offering hope in this area, scientists have developed a new type of battery that incorporates low-cost metal, aluminum, and can efficiently store large amounts of energy.
In a new multi-institutional study, researchers have demonstrated the efficacy of a rechargeable battery that utilizes aluminum and carbon. The battery is said to have a capacity of 10,000 error-free cycles.
"A very interesting feature of this battery is that only two elements are used for the anode and the cathode – aluminum and carbon – both of which are inexpensive and environmentally friendly," said Jingxu Zhen, lead author of the study, in a statement.
Potential Alternative to Lithium-ion Batteries

With no viable options available for the feasible storage of renewable energy, lithium-ion batteries are currently the only choice. However, they are delicate and require protective circuits to safely maintain current and voltage limits. They are expensive to manufacture and slow to recharge. Moreover, they have a tendency to combust.
This is where the new battery, that builds on earlier works of the team, comes into play. In their previous research, the team illustrated the potential of batteries that made use of zinc anodes. For the current study, a new approach was leveraged where they incorporated an aluminum anode in the battery along with a carbon cathode.
"Although superficially different from our earlier innovations for stabilizing zinc and lithium metal electrodes in batteries, the principle is the same – design substrates that provide a large thermodynamic driving force that promotes nucleation; and runaway, unsafe growth of the metal electrode is prevented by forces such as surface tension that can be massive at small scales," said Lynden Archer, senior author of the study.
Tricky Aluminum
Being one of the most widely concentrated elements in the earth's crust, aluminum is easily available. It is light and has a higher energy storage capacity when compared to other metals. Nevertheless, when it comes to its fusing into the electrodes of batteries, aluminum serves as a problematic metal.
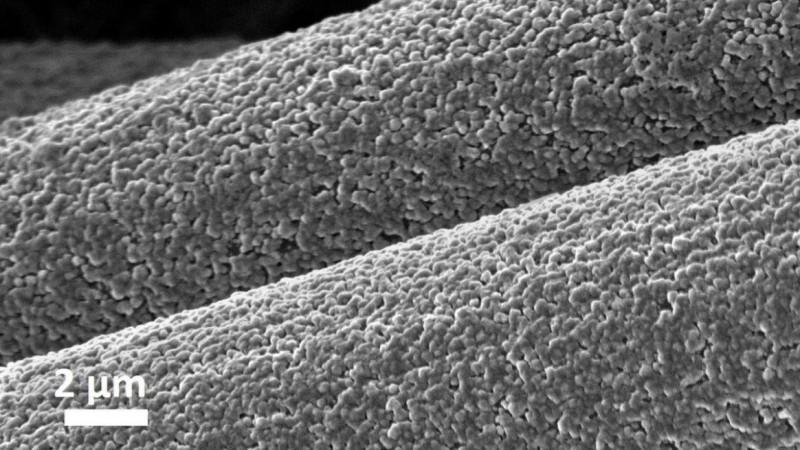
It can chemically react with the glass fiber separator—a structure that divides the anode and the cathode. This can lead to the short-circuiting of the battery and its failure. In order to overcome this hurdle, the authors designed interwoven layers of carbon fiber that could form a powerful chemical bond with aluminum. Upon charging, deposition of aluminum through covalent bonding—sharing of electrons—takes place.
"Basically we use a chemical driving force to promote a uniform deposition of aluminum into the pores of the architecture. The electrode is much thicker and it has much faster kinetics," explained Zheng.
Reduced Cost
The new aluminum-anode batteries have the ability to charged and discharged at magnitudes numerous more times than other rechargeable batteries that utilize aluminum under practical conditions. Along with being cheap and eco-friendly, the materials used in the battery have long life cycles, which result in economical benefits as well.
"When we calculate the cost of energy storage, we need to amortize it over the overall energy throughput, meaning that the battery is rechargeable, so we can use it many, many times. So if we have a longer service life, then this cost will be further reduced," stated Zheng.





!['Had denied Housefull franchise as they wanted me to wear a bikini': Tia Bajpai on turning down bold scripts [Exclusive]](https://data1.ibtimes.co.in/en/full/806605/had-denied-housefull-franchise-they-wanted-me-wear-bikini-tia-bajpai-turning-down-bold.png?w=220&h=138)



