The COVID-19 pandemic seems far from over and shows no signs of subsiding anytime soon. Timely, affordable, and flexible testing is a vital tool in the fight against the SARS-CoV-2 virus. However, despite its efficiency, RT-PCR (Reverse transcription-polymerase chain reaction) test— the gold standard for detecting the novel coronavirus—scores low in the mentioned areas. Presenting a potential alternative, scientists have reported the development of a new test that is both cheaper and easier to use but equally reliable as validated methods.
Designed by researchers from the King Abdullah University of Science and Technology (KAUST), the diagnostic tool called Vigilant is a lateral flow test (much like a commercially available pregnancy test) that utilizes bacterial enzymes with specific genetic sequences and a handful of probes. Along with its g cost-effectiveness and ease of use, the test can identify very low amounts of viral RNA in analyzed samples with very high accuracy.
"Several types of lateral flow tests are currently available or under research for detecting SARS-CoV-2. Depending on how they work, they all have disadvantages, including detecting the virus only several days after infection or producing false-positive and false-negative results. Vigilant can be conducted in nonlaboratory settings and is significantly cheaper and easier to use than PCR tests," said Tin Marsic, co-author of the study, in a statement.
Novel Test for COVID-19

As of now, the RT-PCR test is the most reliable diagnostic technique for detecting the SARS-CoV-2's viral proteins. Its high accuracy and reliability, however, are accompanied by practical limitations that diminish its viability. These include costs, training of professionals, the need for large laboratory infrastructure, and most importantly, lengthy waiting time for results. Results of an RT-PCR test can take anywhere between 24 to 48 hours to be obtained. The design of Vigilant— which stands for VirD2-dCas9 guided and LFA-coupled nucleic acid test—addresses these concerns.
Vigilant's first step involves the utilization of a technique known as reverse transcription-recombinase polymerase amplification (RT-RPA). It helps produce numerous copies of a particular region on the novel coronavirus' genome in the event of viral proteins being present in nasopharyngeal swabs. While PCR/RT-PCR tests also magnify viral genomes present in samples, they require them to be subjected to several low and high-temperature cycles. However, RT-RPA can be conducted at room temperature employing equipment that is much cheaper and easier to use.
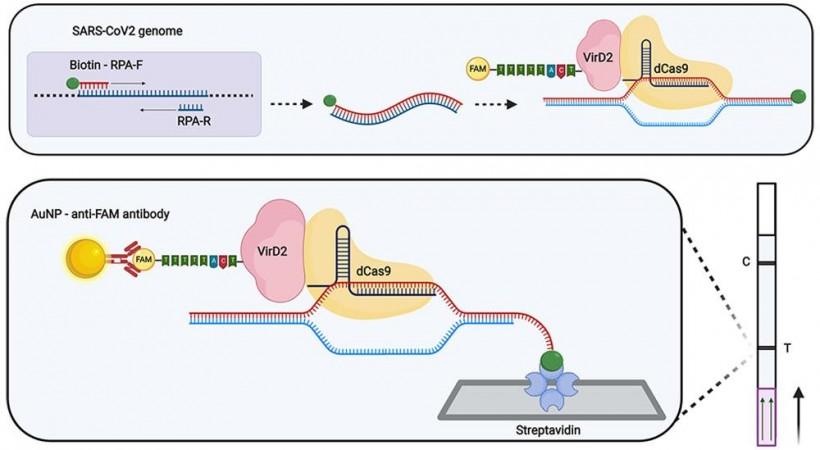
Amplified genes of the SARS-CoV-2 obtained as a product of the first step are tagged with a molecule of biotin—a vitamin—and transferred to a test tube containing a detection complex particularly designed for the test. This special complex comprises two bacterial enzymes that have been modified for Vigilant—Cas9 and VirD2. The Cas9 is combined with an RNA guide that recognizes and attaches itself only to the corresponding gene sequence of the virus. On the other hand, VirD2 is coupled with a fluorescent-tagged nucleotide sequence.
After the reaction between the RT-RPA product and the complex occurs within the test tube, a few drops from the mixture are added to a lateral flow test strip that has been infused with a protein and an antibody. On one end of the strip is streptavidin, a protein that recognizes biotin, and on the other end, is an antibody that can recognize the fluorescent probe.
Spotting the Coronavirus Efficiently
In the event of the sample containing gene sequences of SARS-CoV-2, they will bind with the VirD2-Cas9 complex. A test is said to be positive when two visible lines appear on the strip. The first line is the site of binding between the biotin on the SARS-CoV-2 amplicon (amplified RNA) and streptavidin. While the second line is where the fluorescent tag in the complex binds with the antibody on the strip. However, if a sample tests negative, only one line resulting from the binding between the fluorescent tag and the strip's antibody appears.
In order to eliminate experimental bias within the study, the authors randomized samples (26 positives and 4 negatives), noted results from Vigilant, and compared them to data obtained from RT-PCR tests; validating the new platform. Exhibiting high sensitivity, Vigilant was found to detect 54 out of 56 positive samples. Also, 4 out of 4 negative samples were accurately detected as negative by the novel test. Therefore, the results suggested a 96.4 percent sensitivity and 100 percent specificity (which was in agreement with RT-PCR positive and negative samples respectively).
According to the scientists, the cost of the test is approximately $10/reaction, thereby, making it affordable and scalable in low-resource settings for mass screening of COVID-19 cases. Talking about the furtherance of the testing tool, Dr. Magdy Mahfouz, lead author of the study, stated, "We're now working on making our Vigilant platform more user-friendly by coupling it with an even simpler amplification technique. We are also working on producing other efficient and rapid diagnostic tests that can detect nucleic acids to enable point-of-care testing for pathogens, including viruses and disease markers."

















