The toll that COVID-19 can take on the bodies of healthy individuals with good immunity can be high in several cases. This risk is specially compounded in individuals such as organ transplant recipients or those with Multiple Sclerosis (MS) who are treated with drugs that suppress their immune system. Therefore, the role of vaccination in such groups is of paramount importance. Now, a new study states that patients with MS show robust immune responses to Messenger RNA (mRNA) COVID-19 vaccines
According to a multi-institutional study, patients with MS who undergo anti-CD20 (aCD20) therapy for the management of the disease, showed strong T-cell responses— crucial cells of the immune system—to mRNA vaccines. This was in spite of impaired antibody production resulting from the muting of B cells (that moderate the production) due to aCD20 therapy. The findings were published in the journal Nature Medicine
"The message from this study is clear – it is worthwhile for patients with MS receiving aCD20 treatment to get a COVID-19 vaccine, which will prevent severe illness. Based on this body of evidence, we urge patients with MS receiving aCD20 treatment to get a COVID-19 vaccine if they haven't already," said Dr. E. John Wherry, co-senior author of the study, in a statement.
Muting of B Cells
Multiple sclerosis (MS) is a chronic disease that affects the central nervous system (CNS). It is a condition when the immune system attacks the insulating layers of nerve cells (myelin) in the brain and spinal cord, and damages them.
B cells, or B lymphocytes, are a variety of lymphocytes that play a vital role in the adaptive immune system. They are responsible for mediating the production of antibodies [or antigen-specific immunoglobulin (Ig)] which act against invading pathogens such as viruses and bacteria.
However, in patients with MS, B cells play a significant role in its autoimmune pathogenesis. They moderate this autoimmune response and engage in the development of lesions that plague the CNS. Patients with MS are treated using aCD20 drugs—usually administered every six months—to manage the disease. Since these treatments target B cells, these immune cells are depleted in patients with MS.
While measuring immune responses resulting from COVID-19 vaccination, antibody levels have served as the primary yardstick. However, there is an increasing body of data that shows that T cells also play an important part in the immune response against the SARS-CoV-2. T cells, also known as T lymphocytes and thymocytes, are a type of lymphocytes. They play a crucial role in the adaptive immune system of the body.
Evaluating Immune Responses to mRNA Vaccines
For the study, the authors measured the antibody and T cell responses in 20 individuals afflicted with MS after receiving either the Pfizer–BioNTech or Moderna mRNA vaccines. These patients were undergoing a CD20 treatment. The data from this group was compared to that from a control group (n=10) comprising of healthy individuals. None of the study participants had any prior clinical symptoms or signs of COVID-19.
The plasma and peripheral blood mononuclear cell samples of the participants were examined five times during the course of the study: before their first dose of the mRNA vaccine, 10-12 days after the first dose, before the second dose, 10-12 days after the second dose, and 25-30 days after the second dose.
Varying Antibody Responses
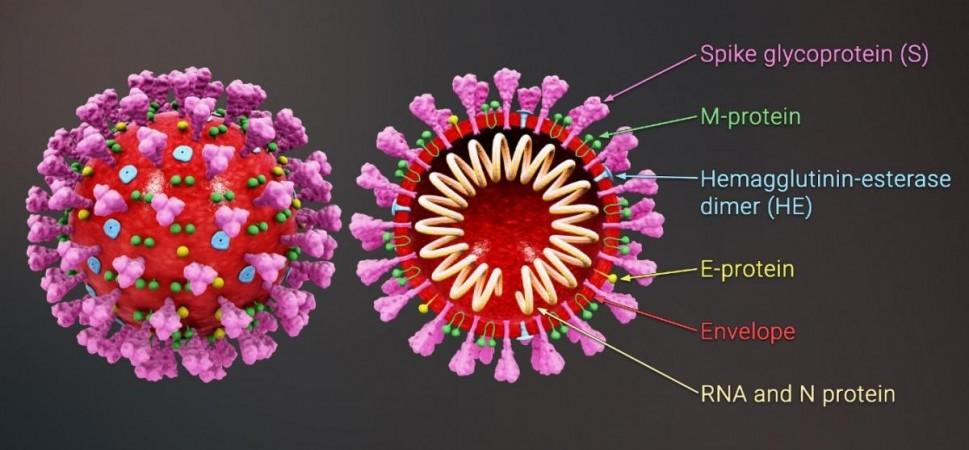
It was noted that in all healthy control subjects, both anti-spike and anti-receptor-binding domain (RBD) antibodies were generated after their first dose of the vaccine. The 'spike' is a hook-like protein structure that the SARS-CoV-2 virus uses to invade cells, while RBD is the area on the spike where the binding with the cells takes place. Also, the levels of these antibodies were found to further increase following the second dose.
However, among MS patients, there were varying antibody responses. 85 percent of participants in the MS group developed antibodies against the SARS-CoV-2 spike, and 50 percent acquired antibody responses against the RBD, by 30 days after the second dose. When compared to the control group, in MS subjects who possessed discernible antibodies, the intensity of the antibody response was generally lesser and delayed.
Importantly, the timing of the last aCD20 infusion that patients in the study received played a crucial role in the immune responses that were triggered. In patients with higher levels of circulating B cells before the vaccine, a stronger antibody response to the vaccine was observed.
Dr. Amit Bar-Or, co-senior author of the study, explained, "This data not only reveal that patients undergoing anti-CD20 infusions are still able to mount important COVID-19 vaccine responses which are likely to protect from severe illness, but also informs our clinical practices in how we advise patients with MS and other autoimmune disorders on such therapies. For example, knowing that responses are weakest immediately following an anti-CD20 infusion, we can now advise patients to wait a number of months after their therapy to get a COVID-19 vaccine."
Robust T Cell Action
The team also gleaned that in patients who had received aCD20 treatments, there were subpopulations of T cells whose response to vaccinations was similar to the healthy subjects in the control group. A robust generation of T cell varieties such as CD4 (helper T cells) and CD8 (killer T cells) was observed as a response to vaccination in patients who had undergone aCD20 therapy.
Additionally, in the subgroup of MS patients who did not produce RBD antibodies, the CD8 T cell response was particularly robust. This finding showed that despite the lack of circulating B cells, the vaccine was able to effectively prime the immune response of the patients towards the coronavirus.
Pointing towards the pitfall of testing only for the presence of antibodies as a measure of immune responses following the administration of an mRNA vaccine, Dr. Sokratis A. Apostolidis, co-lead author of the study, stressed, "Measuring both antibodies and T-cell response gives us a more complete picture of a patient's immune response, and reveals that patients who can't generate antibodies as well as a healthy person are actually still protected by the COVID-19 vaccine."
However, the scientists did admit that on account of the restricted antibody responses triggered in patients undergoing aCD20, their bodies may be unable to neutralize the pathogen as rapidly before the infection of other cells. Due to this, they may be contagious carriers of SARS-CoV-2 for extended periods of time.














