With the desperate need for inoculation against COVID-19 gripping the entire world, vaccines have become an integral part of the common lexicon. The Pfizer-BioNTech vaccine is one such example. However, most people are not aware of the intricate and laborious process that goes into its making.
The Pfizer-BioNTech vaccine received emergency use authorization (EUA) in December 2020 from the US Food and Drug Administration (FDA). It was also the first mRNA vaccine to be given a EUA. So how is this vaccine against the notorious SARS-CoV-2 coronavirus made? It is a complex process that spans over 60 days and requires the utilization of resources at multiple facilities located across two countries. Let us take a look at it.
Step-1: Acquiring DNA from Storage
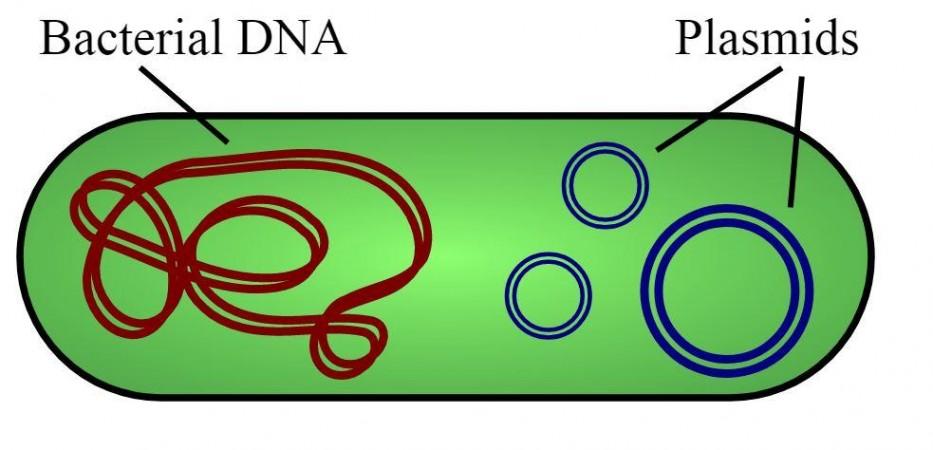
The process begins in Pfizer's facility in Chesterfield, Missouri, where scores of bacteria manufacture mini loops of DNA that contain the SARS-CoV-2's genes. This forms the basic raw material of the vaccine. A vial of DNA (stored at –150°C/–238°F) from the 'master cell bank'—the primary source of all the batches of the company's vaccine— is removed by scientists. Every vial holds DNA molecules known as plasmids.
Every plasmid carries a gene of the coronavirus. This gene serves as the instruction manual for human cells to develop the virus' proteins and initiate an immune response against it. The plasmids are thawed and a modified set of E. coli bacteria absorb the plasmid within their cells. Amazingly, every vial has the potential to help in the production of 50 million doses of the vaccine.
Step-2: Multiplication of Cells
Next, the multiplication and growth of the modified bacteria takes place. The vial containing the microbe is transferred to a flask and shaken in a growth medium, which serves as a warm and sterile environment for growth. It promotes the multiplication of altered bacteria.
Step-3: Fermentation of the Mixture
The reconstructed bacteria are left to grow overnight. Following this, they are shifted to a big fermenter. This structure holds nearly 300 liters of a nutrient-rich substance or 'broth'. The bacteria-laden broth is allowed to ferment for four days. As the bacteria multiply every 20 minutes, trillions of copies of DNA plasmids are produced.
Step 4: Harvesting and Purification of the DNA
Once the fermentation process is over, chemicals are added to the fermented bacterial mixture. These chemicals aid in the rupturing of bacterial cells containing the plasmids, and their release. Now, the mixture undergoes the process of purification in order to eliminate the bacteria and making sure only the precious plasmids are left behind.
Step-5: Assessment of Quality
After purification, the purity of the plasmids is tested. They are evaluated against previously produced samples in order to ascertain whether the sequence of the coronavirus gene has undergone any changes. Any change in the sequence may result in the ineffectiveness of the vaccine. Hence, this is a crucial step.
Step-6: Slicing of the Plasmids
Once the plasmids receive approval for quality, certain enzymes or proteins are introduced into the mixture. The plasmids—which are circular in shape—are cut by the enzymes, and coronavirus genes are isolated into straight segments. Known as linearization, the process takes around two days to achieve completion.
Step-7: Filtration of DNA

Next, the filtration of the mixture takes place in order to ensure that any residual plasmid fragments or bacteria are removed. The resulting purified DNA is left behind in one-liter bottles. The sequences of the DNA are tested one last time. They will act as templates in the following stage of the process. Astonishingly, every bottle of purified DNA can enable the production of around 1.5 million doses of the vaccine each.
Step-8: Frozen, Packed, and Shipped to Other Facilities
Following filtration, every bottle of DNA is frozen, bagged, sealed, and packed. The packaged DNAs also contain a monitor that will log their temperature during transit. With sufficient dry ice to keep them in a frozen state (at –20°C/–4°F), around 48 bottles are packed within a container. With this final step, the action shifts to other facilities.
Talking about the importance of the process at the Chesterfield facility, Sahar Gholami, a laboratory technician at Pfizer, told The New York Times, "What we do is so important because we basically control everything that gets shipped. Every vial that goes to every person goes through us first."
The secured containers are transported to Andover, Massachusetts where a Pfizer manufacturing facility is located. At this site, the DNA is processed into the key ingredient of the vaccine—the messenger RNA (mRNA). Bottles of DNA are also shipped to BioNTech facilities in Mainz, Germany. Here, the DNA will be processed for the European markets and others.
Step-9: Synthesis of mRNA from DNA
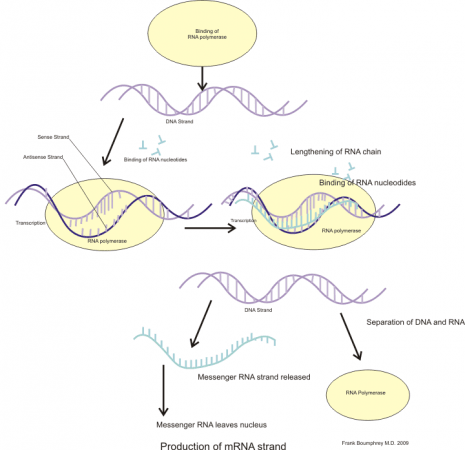
At the Andover plant, transcribing of DNA to mRNA takes place in the mRNA suite. Every day, five bottles of DNA are thawed. They are then mixed with building materials for messenger RNA. The DNA templates are broken open by enzymes and transcribed into mRNA stands over the course of several hours.
This mixture is then transferred to a holding tank. It is then filtered in order to remove unrequired enzymes, DNA, or impurities of any kind. Impressively, every batch can finally result in around 7.5 million doses of the vaccine. When the vaccine is completed and is administered to people, the human cells will receive these mRNA. The cells will identify the SARS-COV-2 gene and commence the production of the virus's protein.
Step-10: Testing of mRNA
The filtered mRNA is then subjected to stringent testing by scientists. This is done in order to confirm its purity and verify the correctness of the genetic sequence. The process results in the production of 10 bags of SARS-CoV-2 mRNA. 16 liters are held in every bag and are the raw material for around 750,000 doses.
Step-11: Frozen, Packed, and Shipped Once More
![Mainz, Germany [in red] (Representational Picture) Mainz](https://data1.ibtimes.co.in/en/full/760775/mainz.jpg?h=450&l=50&t=40)
From the Andover facility, the mRNA bags are sent to a Pfizer facility in Kalamazoo, Michigan after being frozen to –20°C (–4°F). There, the mRNA will be processed into the final product—the vaccine—which will be stored in vials. The Chesterfield facility receives samples which will be tested again.
The production of mRNA at the Andover plant is two batches a week with around 10 bags produced in each. Parallelly, the DNA from the Chesterfield facility is processed in Mainz, Germany, and filtered mRNA bags are sent to a facility in Puurs, Belgium.
Step-12: Preparation of the mRNA
The bags of mRNA shipped from Andover are received at the Kalamazoo facility. Here, the bags are stored in a frozen state until the need for them arises. When needed, the bags are thawed, and the mRNA is mixed with water as a preparatory step for the manufacturing of the vaccine. From every thawed set of mRNA, around 3.6 million doses or 600,000 vials of the vaccine can be produced.

Highlighting the effort going into the production process, Dr. Meg Ruesch, Vice-President for research and development, told The New York Times, "There's no weekend breaks. There's no whitespace in the project plan. You hire people, you put everyone you can on. But the quality built in is the same as we'd do for any vaccine."
Step-13: Preparation of Lipids
Oily lipids that provide the mRNA with protection and aid in its entry into human cells are prepared in a concurrent process. Carefully measured amounts of lipids are mixed with ethanol. This lipid-ethanol mixture will however be eliminated from the completely finished vaccine.
Step-14: Assembling the Vaccine
After traveling across different facilities, the mRNA is finally turned into the final vaccine. Now, the prepared lipid solutions are mixed with the mRNA to create nanoparticles. The flow of the two components is accurately controlled by a rack of 16 pumps. As the lipids make contact with the bare mRNA, they are pulled together by electric charge in a nanosecond.
Numerous layers of lipids envelope the mRNA, and an oily protective vaccine particle is formed as a result. Next, the ethanol is removed from the freshly produced vaccine. The product is concentrated and filtered further in order to do away with any impurities that might have remained. Finally, the vaccine is sterilized.
Step-15: Preparation of Vials

How is the final vaccine stored? In vials of course! Empty vials numbering in hundreds of thousands are washed and subjected to heat sterilization. A high-speed visual inspection is performed by a set of 13 cameras that capture over 100 images of every individual vial. Imperfect and damaged vials are sorted out of the line. The vials are then tested for leaks using vacuum by a separate machine.
Calling attention to the scale of production and the importance of the process, Chaz Calitri, Vice-president of operations, said, "We make hundreds of millions of doses of product going into a lot of people. That's an awesome responsibility, and we take that very, very seriously."
Step-16: Filling the Vials with Vaccine
With the vaccine ready and the vials prepared, filling the vials with the vaccine is the next step. In a narrow single-file line, the flow of the vials begins. 0.45 ml of the concentrated vaccine is injected into each vial by machines. When diluted later, every vial can produce around 6 doses of the vaccines.
Next, the filled vials are foil-sealed and capped using purple lids. This takes place at a stunning pace of around 575 vials per minute. Though the vaccine is chilled, the bottling process can heat up it up rapidly. And if the vaccine remains unfrozen for extended periods of time, the mRNA can degenerate. Therefore, approximately 46 hours is all that the Kalamazoo facility has to fill the vials with liquid vaccine and deep freeze the vials.
Step-17: Packaging, Freezing, and Testing
After one final inspection, the filled vials are labeled and packed into small plastic trays that are informally known as "pizza boxes". Each tray can hold 195 vials. Next, the trays are packed into stacks of five. The stacks are deposited into one of the 350 industrial freezers at the facility. Every freezer can accommodate a whopping 300 trays.
To be able to attain a temperature of –70°C (–94°F) for the purpose of long-term storage, a couple of days are required. Hence, the freezers are individually inspected to make sure that the ultra-cold temperature is maintained. Upon being frozen, the vials of vaccines are kept for four weeks for testing. The Andover facility receives sample vials as it produced the mRNA, and the Chesterfield receives samples as it made the DNA template available.
Step-18: Packing and Shipping of the Finished COVID-19 Vaccine
After undergoing testing for weeks, the finished vaccine is ready for shipping. Trays are removed from the freezers and packed into shipping boxes that are equipped with location and temperature sensors. The minimum number of vials held in a single tray is 195, and up to five such trays are held in a box. As every box requires a massive 45 pounds of dry ice for preservation, the facility produces dry ice on site. Finally, the vaccine is shipped!
.









!['Had denied Housefull franchise as they wanted me to wear a bikini': Tia Bajpai on turning down bold scripts [Exclusive]](https://data1.ibtimes.co.in/en/full/806605/had-denied-housefull-franchise-they-wanted-me-wear-bikini-tia-bajpai-turning-down-bold.png?w=220&h=138)



