The only way to beat a virus is to comprehend all the crucial steps in its life cycle. If this is done successfully, it serves as a vital tool in identifying all the weaknesses that can be exploited to treat the diseases it causes. Now, an international team of researchers has discovered how a virus from the retrovirus family becomes infectious.
In the study, the authors showed how the Rous sarcoma virus (RSV)—which belongs to the same family as the deadly HIV (human immunodeficiency viruses)—protected its genetic information before it finally becomes infectious. The scientists found that a tiny protein particle, IP6, played an important role in the assembly of the virus.
The findings of the study are important as they provide better insights into the closely related HIV, and other retroviruses, and potentially help in the development of better treatments. "If you want to know the enemy, you have to know all its friends," said Dr. Martin Obr, first author of the study in a statement.
A Family of Lethal Viruses
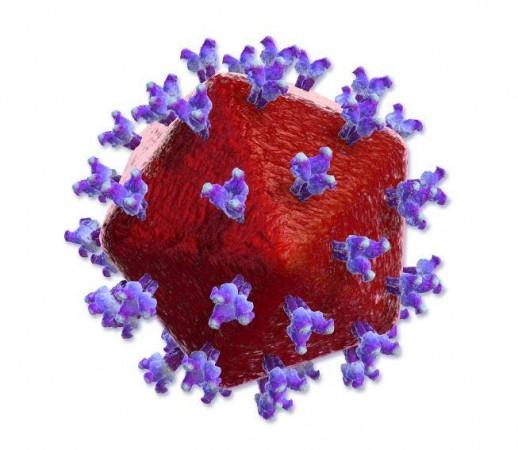
With the SARS-CoV-2 coronavirus keeping the scientific community occupied for 18 months now, research into other lethal viruses has taken a back seat. HIV is one such example. Like HIV, the Rous sarcoma virus is a retrovirus (i.e) it belongs to the family Retroviridae. Retroviruses are viruses whose genetic material is RNA.
When they infect a host cell, they make a DNA copy (or form) of their RNA which inserts itself within the DNA of the cell. Following this, the infected cell begins treating the new DNA as its genetic material and producing viral proteins of the infecting virus, or in other words, becoming a factory for the virus' replication. While HIV affects human beings, the RSV affects chickens and leads to the development of cancer in them. RSV is also the first discovered oncovirus (i.e) a virus that triggers cancer.
However, the mechanism of infection of both viruses is the same. Thus, insights into the poultry virus can enable breakthroughs in the further understanding of HIV. Often, HIV infection results in the development of AIDS (Acquired immunodeficiency syndrome). According to WHO, around 38 million people were said to be living with HIV at the end of 2019. In many ways, HIV infection has been a pandemic that has persisted for over three decades.
Discovering Key to Infectivity
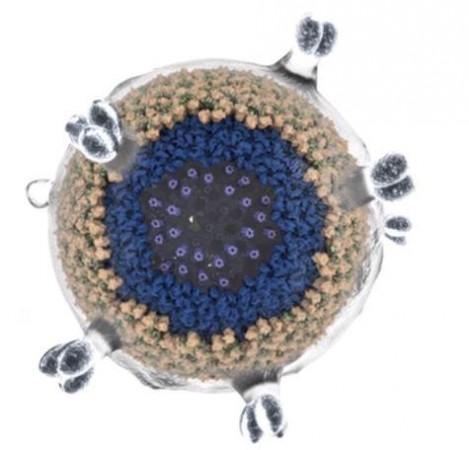
For the study, the authors concentrated on the late phase of retrovirus replication. A particle that emerges from an infected cell is in an immature and non-infectious state. "It is a long way from an infected cell to the mature virus particle that can infect another cell," explained Obr. Next, the viral particle develops a protein shell around it called a capsid. This protein is organized as hexamers (a molecule composed of six structural subunits) and few pentamers (a molecule composed of five structural subunits).
It was discovered that a small molecule known as IP6 plays a crucial role in the stability of the protein shell within the Rous sarcoma virus. Dr. Florian Schur, corresponding author of the study, illustrated: "If the protective shell is not stable, the genetic information of the virus could be released prematurely and will be destroyed." However, high stability would completely prevent the exit of the genome and therefore render it useless, he highlighted. In an older study, Dr. Schur and his colleagues had proved that IP6 was important in the assembly of the HIV virus.
Through the current study, the team discovered and proved that IP6 was equally important in other retroviruses, and evidenced the essential role that the tiny molecule played in the virus life cycle. Stressing on its importance, Dr. Orb remarked, "When building a car, you have all these big metal parts, like the hood, the roof and the doors – the screws are connecting everything. In our case, the big parts are the capsid proteins and the IP6 molecules are the screws."
An Unexpected Customization
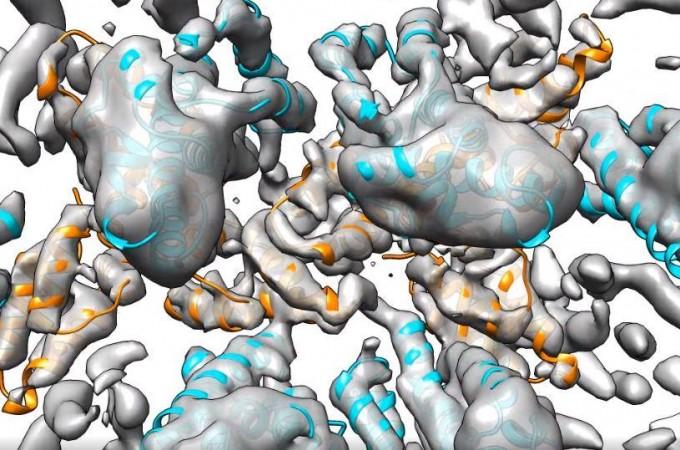
Cryo-electron tomography is an imaging technique that enables researchers to see particles in extremely small samples in their natural state. Using this method, and developing on it further, the scientists were able to observe how the capsid proteins formed in varying shapes. The formation of different capsid shapes within the same type of virus could indicate the differing infectivity of the viral particles.
Dr. Obr expressed, "Now we ask ourselves: Why does the virus change the shape of its capsid? What is it adapting to?" Dr. Schur noted that such differences were not without a reason. However, there is no clear clarification for it yet, he concluded. The future development of the imaging technique could perhaps help scientists get a deeper look into these highly-optimized viruses.









