On the day envoys of 64 countries landed in India to visit the Hyderabad-based Bharat Biotech's facility, a media report claimed that the company's proposal for the emergency use of its COVID-19 vaccine has been rejected.
Bharat Biotech is developing a vaccine called Covaxin in collaboration with the Indian Council of Medical Research (ICMR).
The report further stated that the novel coronavirus vaccine being manufactured by the Serum Insitute of India, the world's largest vaccine manufacturer, has also failed to receive the nod for emergency use.
Emergency use refers to the authorisation is a mechanism to facilitate the availability and use of medical countermeasures, including vaccines, during public health emergencies.
The claim
According to NDTV, the Drugs Controller General of India (DCGI) on Wednesday rejected the applications filed by Serum Institue of India and Bharat Biotech for emergency use authorisation of their respective COVID-19 vaccines.
"Both proposals are not approved due to inadequate safety and efficacy data available currently. Both have been asked for more data," NDTV quoted sources as saying.
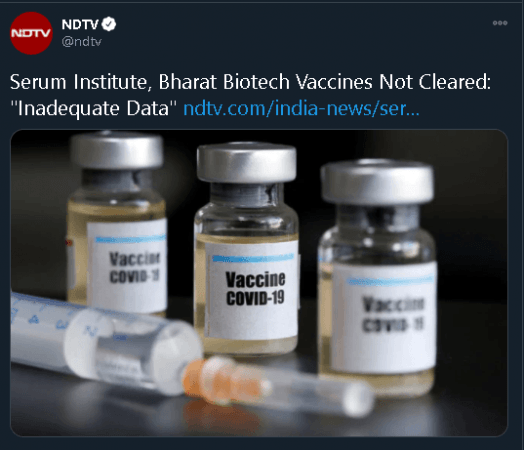
The Serum Institute of India had on Sunday applied for an emergency use license for its COVID-19 vaccine that it is developing in collaboration with the University of Oxford and British drugmaker AstraZeneca, whereas Bharat Biotech sent its proposal a day after.
Fact-check
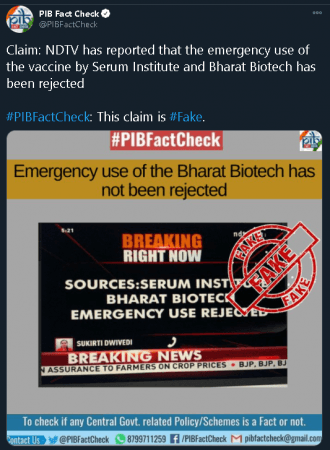
The Ministry of Health and Family Welfare (MoHFW) categorically denied the claims made by NDTV as it dubbed the reports of Serum Institute of India and Bharat Biotech's proposals getting rejected as false.
Sharing a screenshot of the aforementioned report, the MoHFW via its official Twitter account said, "#FAKENEWS The news running on @ndtvindia is Fake News."
Subsequently, the fact-checking arm of the Press Information Bureau (PIB) also put out a clarification in regards to the report claiming rejection of the Serum Institute of India and Bharat Biotech's COVID-19 vaccines.
"The media report about the rejection of Serum Institute and Bharat Biotech's emergency use authorisation of vaccine is fake: Ministry of Health & Family Welfare," tweeted PIB Fact Check.
Covaxin, which has been evaluated in 1,000 subjects in Phase I and Phase II clinical trials with promising safety and immunogenicity data, has been pitched as India's first indigenous vaccine candidate.
On November 28, Prime Minister Narendra Modi had visited the Bharat Biotech facility in Hyderabad to review vaccine development. "At the Bharat Biotech facility in Hyderabad, was briefed about their indigenous Covid-19 vaccine. Congratulated the scientists for their progress in the trials so far. Their team is closely working with ICMR to facilitate speedy progress," the PM had tweeted.

















