When it comes to testing for COVID-19, time is life. The sheer number of new infections in countries such as India mandate diagnostic tests that are both rapid and scalable. Now, scientists from the Hokkaido University have demonstrated the simplicity and accuracy of a rapid-antigen test that can detect the SARS-CoV-2 coronavirus in saliva samples within 35 minutes.
In the study, the authors showed the efficiency of chemiluminescent enzyme immunoassay (CLEIA), using an antigen-based testing kit known as Lumipulse, in rapidly detecting the novel coronavirus. They also compared CLEIA with RT-PCR, or RT-qPCR—the gold standard in COVID-19 diagnosis—and evidenced that the former was highly accurate.
"Our results showed high correlation between antigen concentrations measured by CLEIA and RNA load measured by RT-qPCR, indicating CLEIA to be a reliable and accurate test," the authors wrote.
Need for Speed
Well into the second year of the COVID-19 pandemic, Reverse transcription-polymerase chain reaction (RT-PCR) continues to remain the most reliable test for the diagnosis of the viral disease. However, despite its accuracy, it presents several practical hurdles, especially when time is of the essence. Some of its limitations are its cost, training of professionals, need for large laboratory infrastructure, and most importantly, long waiting time for results. It can take anywhere between 24 to 48 hours to acquire the results of an RT-PCR test.
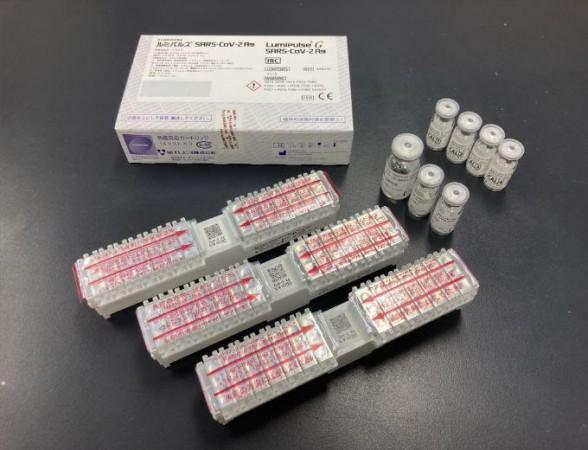
As scientists across the world work/worked towards developing quicker yet equally reliable tests, Fujirebio, a Japanese company involved in clinical diagnostics, created the Lumipulse SARS-CoV-2 Ag kit (Lumipulse) in June 2020. The antigen-based test measures the viral antigen in biological samples quantitatively in less than 35 minutes. It can detect SARS-CoV-2 nucleoproteins in nasopharyngeal swabs and saliva samples.

In the current study, the authors used the kit to test CLEIA's viability. CLEIA is an immunoassay technique that utilizes luminescent compounds that emit light during the course of enzymatic chemical reactions. It has high specificity and sensitivity. Even nearly negligible amounts of biological molecules can be detected using CLEIA. Therefore, the research team tested its potential to rapidly identify SARS-CoV-2.
Accurate and Reliable
For the study, the authors analyzed saliva samples of 2056 individuals from three cohorts. The three groups of participants were: patients who were clinically confirmed to have COVID-19; individuals who were tested upon arrival at Kansai and Tokyo International Airports; and individuals who had been in contact with COVID-19 patients.
The collected saliva samples were subjected to both RT-PCR tests as well CLEIA (using Lumipulse). Results from both the tests were analyzed and compared. It was found that the results from CLEIA correlated well with RT-PCR, thus, proving its reliability. Using CLEIA alone, SARS-CoV-2 can be detected in under 35 minutes.
Combined Testing for Better Diagnosis

Due to the use of only saliva for testing, the new testing technique makes the collection of samples simple and painless. It also reduces the risk of exposure for healthcare workers whose expertise in testing methods also puts them in dangerously close proximity to the virus. However, the most remarkable advantage of the test is the ease of self sample collection, and large-scale sample collection as well.
While the researchers illustrated the speed and reliability of CLEIA, they emphasized that a combination of CLEIA and RT-PCR can improve accuracy. According to the scientists, employing CLEIA for screening, and RT-PCR for confirmation increases the precision of the diagnosis.
The combined testing methodology is already in use at Japanese airport quarantines, and the team calls for its adoption on a larger scale for rapid screening and isolation. "To improve accuracy, we propose a two-step screening strategy with an initial CLEIA test followed by confirmatory RT-qPCR for intermediate concentrations, varying positive and negative thresholds depending on local prevalence," the authors wrote.









!['Had denied Housefull franchise as they wanted me to wear a bikini': Tia Bajpai on turning down bold scripts [Exclusive]](https://data1.ibtimes.co.in/en/full/806605/had-denied-housefull-franchise-they-wanted-me-wear-bikini-tia-bajpai-turning-down-bold.png?w=220&h=138)



