An FDA approved blood disorder drug can help regrow hair in people with a rare hair loss condition, reveals a new study.
Three patients, affected with alopecia areata, were completely cured after they took ruxolitinib, a drug approved for blood disorder myelofibrosis.
Alopecia areata is an auto immune disease that leads to loss of hair in round patches, mainly on the scalp. However, it can also affect face or any other parts of the body.
"We've only begun testing the drug in patients, but if the drug continues to be successful and safe, it will have a dramatic positive impact on the lives of people with this disease," researcher Dr Raphael Clynes, from the Columbia University Medical Center in New York, said in a news release.
The disease occurs when certain immune cells attack and destroy hair follicles. However, until the date, researchers were not clear about the main culprit or the specific type of immune cells behind the disease.
Researchers based their findings on a previous study that exposed a "danger signal" in the hair follicles that triggers the immune response to attack the hair follicle. They conducted experiments on mice models to identify the T cells behind the attack. Using mouse and human cells with the condition, researchers exposed the whole mechanism and found JAK inhibitors, a class of drugs highly effective in fighting this condition.
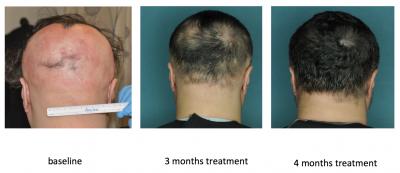
Two drugs ruxolitinib and tofacitinib succeeded in inhibiting the immune pathways and blocking the immune attack. In animals, these drugs helped regrow hair within three months and remained effective even after discontinuing the treatment.
Later, researchers analysed effect of ruxolitinib on patients who had more than 30 percent hair loss. Interestingly, the drug restored hair growth in three patients within four to five months starting the treatment. A closer examination conducted after the treatment also showed that T cells responsible for the disease had completely vanished from the scalp.
"We still need to do more testing to establish that ruxolitinib should be used in alopecia areata, but this is exciting news for patients and their physicians," Dr. Clynes said. "This disease has been completely understudied—until now, only two small clinical trials evaluating targeted therapies in alopecia areata have been performed, largely because of the lack of mechanistic insight into it."
Results of the study reported in Nature Medicine, reflect another study that appeared in the Journal of Investigative Dermatology in June. Brett A. King, assistant professor of dermatology at Yale University School of Medicine, in US, cured an alopecia universalis patient with tofacitnib citrate. Alopecia universalis is a medical condition that leads to hair loss in the entire body, including the scalp, eyebrows and eyelashes.














