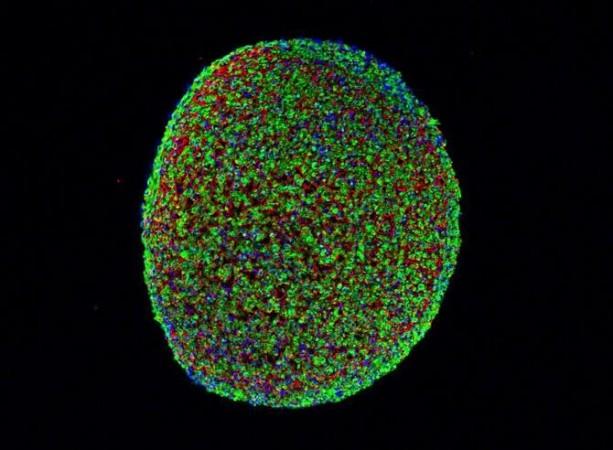
A team of researchers from Duke University has successfully grown the first-ever working human skeletal muscle from pluripotent stem cells, cells that can give rise to any cell type in the body.
The work, details of which were published in the journal Nature Communications on Tuesday, builds on a 2015 research, in which the scientists created the first functioning human muscle tissue from cells obtained from muscle biopsies, called "myogenic precursors."
The researchers working on the research now expect that the ability to grow functioning muscles from cellular scratch using non-muscle tissue will pave the way for creating far more muscle cells while providing easier ways to edit genome and develop better cellular therapies.
Scientists also expect the breakthrough to help them grow individually tailored models of rare muscle diseases for drug discovery as well as for better understanding of human biology.
"Starting with pluripotent stem cells that are not muscle cells, but can become all existing cells in our body, allows us to grow an unlimited number of myogenic progenitor cells," Nenad Bursac, professor of biomedical engineering at Duke University, said in a statement.
"These progenitor cells resemble adult muscle stem cells called 'satellite cells' that can theoretically grow an entire muscle starting from a single cell," Bursac added.
As part of the new study, the researchers used pluripotent stem cells taken from adult non-muscle tissues like skin or blood. These cells were then "reprogrammed" to revert to a primordial state when they were much simpler and undefined.
To initiate the stem cells' transformation into muscle cells, the researchers used a molecule called Pax7 which signals the cells to start becoming muscle. As the cells grew, they became very similar to adult muscle stem cells.
While previous studies were successful only until this point, the new research yields the first evidence showing intermediate cells turning into functioning skeletal muscle.
"It's taken years of trial and error, making educated guesses and taking baby steps to finally produce functioning human muscle from pluripotent stem cells," Lingjun Rao, a postdoctoral researcher in Bursac's laboratory and first author of the study, said.
"What made the difference are our unique cell culture conditions and 3-D matrix, which allowed cells to grow and develop much faster and longer than the 2-D culture approaches that are more typically used," Rao said.
To check how functional the stem cell-grown tissue was, the researchers implanted it into adult mice and found that it survived and effectively functioned as it was gradually integrated into the native tissue for at least three weeks.
The study, however, also revealed that the resulting muscle was not as strong as native muscle tissue. Despite the drawback, the muscle still holds more potential than its stronger counterpart as it contains more "satellite-like cells" that are needed for normal adult muscles to repair damage.
"When a child's muscles are already withering away from something like Duchenne muscular dystrophy, it would not be ethical to take muscle samples from them and do further damage. But with this technique, we can just take a small sample of non-muscle tissue, like skin or blood, revert the obtained cells to a pluripotent state, and eventually grow an endless amount of functioning muscle fibres to test," Bursac said.













!['It's not Mumbai traffic, it's air traffic': Suriya apologises to Mumbai media after paparazzi yelled At Him for making them wait for hours [Watch]](https://data1.ibtimes.co.in/en/full/806234/its-not-mumbai-traffic-its-air-traffic-suriya-apologises-mumbai-media-after-paparazzi.jpg?w=220&h=138)



