It is a known fact that as we age, our muscle health deteriorates. Along with becoming weaker and smaller, the ability of muscles to heal themselves after an injury also declines with age. What if there was a way of reversing this process? Offering hope for future therapies that can help achieve this, scientists have discovered a crucial factor that aids in the rejuvenation of aged muscles.
Through a new multi-institutional study, researchers have demonstrated that molecules known as extracellular vesicles (EVs) carry genetic instructions (mRNA) for the vitality of an anti-aging protein called α-Klotho (Klotho) to muscle cells. The animal study showed that impaired muscle repair and loss of muscle function in older mice may be guided by aged EVs, which bear fewer copies of mRNA than younger ones.
"In one way, it helps us understand the basic biology of how muscle regeneration works and how it fails to work as we age. Then, taking that information to the next step, we can think about using extracellular vesicles as therapeutics to counteract these age-related defects," said Dr. Fabrisia Ambrosio, senior author of the study, in a statement. The findings were published in the journal Nature Aging.
Delivering Crucial 'Cargoes'

Extracellular vesicles (EVs) are a heterogeneous collection of membrane-bound particles that are secreted in the extracellular space by different types of cells. They act as shuttles or carriers of complex cargoes such as lipids, nucleic acids, and proteins. Research over the decades has shown that youthful characteristics are restored to several cells and tissues of aged mice when they are given blood from young mice.
The current study is built on these learnings. "We wondered if extracellular vesicles might contribute to muscle regeneration because these couriers travel between cells via the blood and other bodily fluids. Like a message in a bottle, EVs deliver information to target cells," explained Dr. Amrita Sahu, lead author of the study.
Regeneration through 'Young' Blood
For the study, the team collected serum—the fluid that remains following the removal of blood cells and clotting factors such as fibrinogen and prothrombin—from young mice. This serum was injected into aged mice who had muscle injuries. Amazingly, amplified muscle regeneration and functional recovery were noted in mice who had received young serum when compared to mice who were administered a placebo.
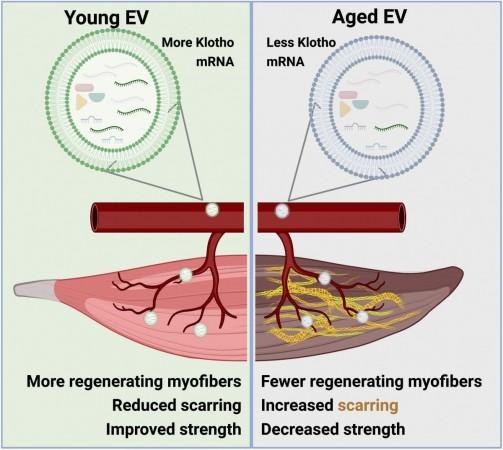
However, when the EVs were depleted or removed, the curative properties of the serum were lost or declined. This suggested that these vesicles could be mediating the advantageous effects within young blood. Further exploration revealed that messenger RNA (mRNA)—RNA molecules carrying genetic instructions—that encode the anti-aging protein Klotho were delivered to skeletal muscle progenitor cells (SMPCs) by EVs.
SMPCs are a variety of stem cells that play a key role in the regeneration of skeletal muscles. EVs obtained from aged mice were found to contain lesser copies of mRNA from Klotho when compared to those from young mice, causing SMPCs to produce a lesser quantity of the protein.
Potential for Future Therapies

The new study, therefore, is the first to demonstrate that age-related changes in EV delivery are instrumental in the depletion of Klotho in aged muscle stem cells. This suggests that novel therapies for the healing of damaged muscle tissues can be based on EVs.
In their previous work, some of the authors involved in the study demonstrated that Klotho is a key regulator of the healing capacity of SMPCs and that the protein decrease with age. The application of EVs may also extend beyond muscles such as reversing the effects of aging. Older studies have evidenced the boosting of cognitive performance in aged mice using young blood.
Illustrating the potential of EV-driven therapies, Dr. Ambrosio stated, "EVs may be beneficial for boosting regenerative capacity of muscle in older individuals and improving functional recovery after an injury. One of the ideas we're really excited about is engineering EVs with specific cargoes, so that we can dictate the responses of target cells."

















