After the Jammu branch of the Council of Scientific and Industrial Research-Indian Institute of Integrative Research (CSIR-IIIM) has demonstrated that the 'Niclosamide' is effective for the treatment of COVID, CSIR India in collaboration with Laxai Life Sciences Pvt. Ltd has initiated a phase-II clinical trial of this drug.
Collaborative research between CSIR-IIIM, Jammu, and National Centre for Biological Science (NCBS), Bangalore has recently demonstrated that Niclosamide is also a potential SARS-CoV-2 entry inhibitor blocking the viral entry through the pH-dependent endocytic pathway.
Niclosamide earlier used for treatment of tapeworm's infection
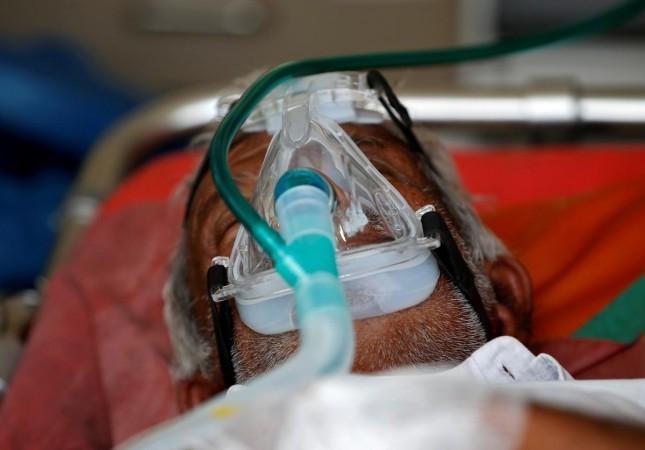
Niclosamide has been extensively used in past for the treatment of tapeworm infection in adults as well as children. The safety profile of this drug has been tested over time and has been found safe for human consumption at different dose levels.
As per an official handout issued by the ministry of science and technology, in a screen to identify drugs that can inhibit syncytia formation, Niclosamide was identified as a promising repurposed drug by a research group from King's College, London, who collaborated in this project. The syncytia or fused cells observed in the lungs of patients with COVID probably result from the fusogenic activity of the SARS-CoV-2 spike protein and Niclosamide can inhibit syncytia formation.
Niclosamide is generic, affordable drug
Dr. Shekhar C Mande, Director General, CSIR expressed his happiness over the SEC recommendations to conduct this phase II clinical trial using Niclosamide, which is a generic, affordable drug and easily available in India and therefore can be made available to our population.
Realizing the potential of Niclosamide, efforts were initiated last year itself to undertake clinical trials. Having received approval from the drug regulator, the clinical trial has been initiated this week at different sites, and is expected that the trial will be completed within 8-12 weeks. Based on successful clinical evidence generated during clinical trials in Indian studies, emergency use authorization may be sought so that more treatment options are available to COVID patients.














